LENZ Therapeutics, Inc. - Annual Report: 2022 (Form 10-K)
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
WASHINGTON, DC 20549
FORM 10-K
(Mark one)
☒ ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the fiscal year ended December 31, 2022
OR
☐ TRANSITION REPORT PURSUANT TO SECTION 13 OR 15 (d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the transition period from to
Commission File Number: 001-40532
GRAPHITE BIO, INC.
(Exact name of Registrant as specified in its charter)
|
|
|
Delaware |
2836 |
84-4867570 |
(State or other jurisdiction of incorporation or organization) |
(Primary Standard Industrial Classification Code Number) |
(I.R.S. Employer Identification No.) |
201 Haskins Way, Suite 210
South San Francisco, CA, 94080
(650) 484-0886
(Address, including zip code and telephone number, including area code, of Registrant’s principal executive offices)
Securities registered pursuant to Section 12(b) of the Act:
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
Common Stock, par value $0.00001 per share |
|
GRPH |
|
The Nasdaq Global Market |
Securities registered pursuant to Section 12(g) of the Act:
None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐ No ☒
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. Yes ☒ No ☐
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files). Yes ☒ No ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company,” and “emerging growth company” in Rule 12b-2 of the Exchange Act.
Large accelerated filer |
|
☐ |
|
Accelerated filer |
|
☐ |
|
|
|
|
|||
Non-accelerated filer |
|
☒ |
|
Smaller reporting company |
|
☒ |
|
|
|
|
|
|
|
Emerging growth company |
|
☒ |
|
|
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report. ☐
If securities are registered pursuant to Section 12(b) of the Act, indicate by check mark whether the financial statements of the registrant included in the filing reflect the correction of an error to previously issued financial statements. ☐
Indicate by check mark whether any of those error corrections are restatements that required a recovery analysis of incentive-based compensation received by any of the registrant’s executive officers during the relevant recovery period pursuant to §240.10D-1(b). ☐
As of June 30, 2022, the aggregate market value of the registrant’s common stock held by non-affiliates of the registrant was approximately $150.1 million, based on the closing price of the registrant’s common stock on the Nasdaq Global Market of $2.75 per share.
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Exchange Act). Yes ☐ No ☒
As of March 14, 2023, the registrant had 58,163,637 shares of common stock, $0.00001 par value per share, outstanding.
DOCUMENTS INCORPORATED BY REFERENCE
Portions of the registrant’s Definitive Proxy Statement relating to its 2023 Annual Meeting of Stockholders (the “Proxy Statement”), which the registrant intends to file pursuant to Regulation 14A with the Securities and Exchange Commission (the “SEC”) not later than 120 days after the registrant’s fiscal year end of December 31, 2022 or an amendment on Form 10-K/A filed with the SEC within 120 days after the end of the registrant’s fiscal year, are incorporated by reference into Part III of this Annual Report on Form 10-K where indicated.
Table of contents
2
Cautionary Note Regarding Forward-Looking Statements
This Annual Report on Form 10-K (this “Form 10-K”), including its section entitled “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” contains express or implied forward-looking statements that are based on our management’s belief and assumptions and on information currently available to our management. Although we believe that the expectations reflected in these forward-looking statements are reasonable, these statements relate to future events or our future operational or financial performance, and involve known and unknown risks, uncertainties, and other factors that may cause our actual results, performance, or achievements to be materially different from any future results, performance, or achievements expressed or implied by these forward-looking statements. Forward-looking statements in this Form 10-K may include, but are not limited to, statements about:
In some cases, forward-looking statements can be identified by terminology such as “will,” “may,” “should,” “could,” “expects,” “intends,” “plans,” “aims,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “continue,” or the negative of these terms or other comparable terminology, although not all forward-looking statements contain these words. These statements are only predictions. You should not place undue reliance on forward-looking statements because they involve known and unknown risks, uncertainties, and other factors, which are, in some cases, beyond our control and which could materially affect results. Factors that may cause actual results to differ materially from current expectations include, among other things, those listed under the section entitled “Risk Factors” and elsewhere in this Form 10-K. If one or more of these risks or uncertainties occur, or if our underlying assumptions prove to be incorrect, actual events or results may vary significantly from those expressed or implied by the forward-looking statements. No forward-looking statement is a promise or a guarantee of future performance.
3
The forward-looking statements in this Form 10-K represent our views as of the date of this Form 10-K. We anticipate that subsequent events and developments will cause our views to change. However, while we may elect to update these forward-looking statements at some point in the future, we have no current intention of doing so except to the extent required by applicable law. You should therefore not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this Form 10-K.
This Form 10-K may include statistical and other industry and market data that we obtained from industry publications and research, surveys, and studies conducted by third parties. Industry publications and third-party research, surveys, and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. We have not independently verified the information contained in such sources.
We use various trademarks and trade names in our business, including without limitation our corporate name and logo. All other trademarks or trade names referred to in this Form 10-K are the property of their respective owners. Solely for convenience, the trademarks and trade names in this Form 10-K may be referred to without the ® and ™ symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto.
Summary of Risk Factors
Our business is subject to a number of risks of which you should be aware before making an investment decision. These risks are discussed more fully in the “Risk Factors” section of this Form 10-K. These risks include, but are not limited to, the following:
4
5
PART I
Item 1. Business.
Overview
We are a clinical-stage, next-generation gene editing company harnessing high-efficiency targeted gene integration to develop a new class of therapies to potentially cure a wide range of serious and life-threatening diseases. Our precision gene editing approach aims to achieve one of medicine’s most elusive goals: to precisely “find & replace” any gene in the genome. We have a next-generation gene editing platform that is designed to precisely correct mutations, replace entire disease-causing genes with normal genes, or insert new genes into predetermined, safe locations. We believe our approach could enable broad applications to transform human health, including directly correcting mutations, engineering cells to permanently deliver therapeutic proteins, and precisely engineering effector cells to treat or cure a wide range of serious genetic and other diseases, including cancer, autoimmune and neurodegenerative diseases.
In January 2023, we announced a voluntary pause of our Phase 1/2 CEDAR study of our lead product candidate nulabeglogene autogedtemcel (nula-cel, formerly GPH101) for sickle cell disease (SCD) due to a serious adverse event in the first patient dosed, which we concluded is likely related to study treatment. In February 2023, we announced our decision to discontinue the development of nula-cel and initiate a process to explore strategic alternatives. Nula-cel was designed to provide a highly differentiated approach with the potential to directly correct the mutation that causes sickle cell disease (SCD) and restore normal adult hemoglobin (HgbA) expression. Curing sickle cell disease by correcting the disease-causing point mutation to normal is viewed as the gold-standard for curing SCD and has been the dream of treating physicians for generations.
Our technology builds on first-generation proven CRISPR technology to achieve high rates of targeted gene integration. Our platform technology includes patent rights and proprietary technology exclusively licensed from The Board of Trustees of the Leland Stanford Junior University (Stanford) and developed in the Stanford laboratories of two of our scientific founders, both pioneers in gene therapy and gene editing: Matthew Porteus, M.D., Ph.D., and Maria Grazia Roncarolo, M.D. Dr. Porteus is considered to be one of the founders of the field of gene editing and was a scientific founder of CRISPR Therapeutics AG. He was the first to demonstrate that an engineered nuclease could be used to correct genes by harnessing precision cellular DNA repair machinery. Dr. Roncarolo is a pioneer in multipotent hematopoietic stem cell (HSC) gene therapy and her work led to the first approved HSC gene therapy product. She established and is Director of the Stanford Center for Definitive and Curative Medicine to treat patients with currently incurable diseases through the development of innovative stem cell- and gene-based therapies. Drs. Porteus and Roncarolo, both practicing physicians, came together with the conviction that targeted gene integration could lead to an entirely new class of potentially curative therapies
.
Our approach has broad therapeutic applications and has enabled high-efficiency targeted gene integration in a wide range of primary human cell types. In our initial programs, we applied our approach ex vivo in a patient’s own HSCs which are reinfused after gene integration (autologous HSCT). HSCs are multipotent stem and progenitor cells that can give rise to all cells of the blood and immune system and have proven their curative potential across dozens of diseases as demonstrated by allogenic HSC transplant (allo-HSCT).
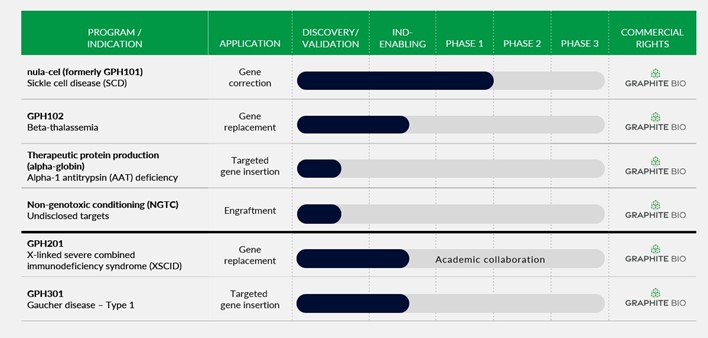
Our approach can be thought of as “find & replace,” using CRISPR to find a target gene and homology directed repair (HDR) to replace DNA in the target gene with DNA copied from a template. We create a precise incision in a target gene using a modified, high fidelity CRISPR-based nuclease and then induce conditions in target cells that overwhelmingly favor HDR, a natural and precise cellular DNA repair process. Using a non-integrating AAV6 vector, we deliver a donor DNA template strand to the target gene which is copied via HDR to create a new coding strand. We then apply our HSC biology expertise to optimally engineer and manufacture HSCs, a historically intractable cell type for harnessing HDR. Using our next-generation gene editing approach, we have achieved gene integration efficiencies in excess of expected curative thresholds and demonstrated preclinical proof-of-concept across models of multiple diseases. Beyond nula-cel, which leverages our gene correction technology, we have previously pursued multiple programs such as GPH102 for beta-thalassemia and GPH201 for X-linked severe combined immunodeficiency syndrome (XSCID), which leveraged our gene replacement technology; GPH301 for Gaucher disease and an early-stage research program for alpha-1 antitrypsin (AAT) deficiency, which leveraged our targeted gene insertion technology; and multiple undisclosed programs in both HSCs and other cell types. Given these programs use the same gene editing platform technology as nula-cel, we do not currently intend to continue development of these programs
.
Our approach differs from first generation gene and base editing technologies due to:
6
We believe our technology can be applied in the following three key settings: Gene Correction, Gene Replacement, and Targeted Gene Insertion.

Gene Correction
Our approach is designed to allow us to precisely correct pathogenic genes by directly targeting and correcting the specific disease-causing mutation to restore the normal, wild-type sequence.
In January 2023, we announced a voluntary pause of our Phase 1/2 CEDAR study of nula-cel, our lead product candidate for sickle cell disease (SCD), due to a serious adverse event in the first patient dosed, which we concluded is likely related to study treatment. In February 2023, we announced our decision to discontinue the development of nula-cel and initiate a process to explore strategic alternatives.
Nula-cel is designed to directly correct the genetic mutation responsible for SCD. The mortality and morbidity associated with SCD, all caused by a single mutation, has made curing SCD a dream of many clinicians. Multiple genetic therapies are in development to address SCD, but due to technical limitations, these therapies are primarily focused on expressing alternate hemoglobin genes such as fetal hemoglobin (HgbF) or a transgenic hemoglobin. Our approach is the first in industry to directly correct the SCD-causing mutation by replacing it with the natural genetic sequence to thereby reduce harmful sickle hemoglobin (HgbS) production and restore normal adult hemoglobin (HgbA) expression. We have optimized our process to correct the majority of HSPCs. Of the remaining cells, which are not corrected, many contain two INDEL sickle globin alleles (knockout alleles). These knockout stem cells are not able to produce sickle red blood cells, and have the effect of increasing the proportion of functional stem cells which have been corrected. This increases
7
our confidence in our ability to exceed the 20% expected curative threshold in patients. Under IND-enabling GMP manufacturing conditions, we can precisely correct the SCD mutation in over 55% of treated cells, which we believe can achieve the threshold required to cure patients (estimated to be engraftment of 20% corrected cells). These treated HSPCs are fully functional and can engraft in vivo in a humanized mouse, and can produce functionally normal red blood cells expressing normal adult hemoglobin ex vivo. Furthermore, we have demonstrated in a mouse model of SCD that our approach significantly increased normal HgbA expression, reduced HgbS production, extended red blood cell (RBC) lifespan from two days in sickle mice to up to 19 days in gene corrected mice, and eliminated RBC sickling. We believe this data supports the curative potential of our approach.
Gene Replacement
Our gene replacement approach is designed to allow us to replace dysfunctional genes with a new normal copy of an entire gene at its normal location in the chromosome. We had initiated two gene replacement programs, one for beta-thalassemia and another for XSCID. Given these programs use the same gene editing platform technology as nula-cel, we do not currently intend to continue development of these programs mentioned below.
Targeted Gene Insertion
Our technology aims to enable the targeted insertion of entire gene cassettes into chosen chromosomal locations. We believe that this could have broad therapeutic applications by allowing for permanent production of therapeutic proteins and enzymes, in specific cell lineages, and from targeted genomic locations. This approach was designed to prevent the variability in gene expression and the potential risk of insertional oncogenesis which are limitations of random gene integration approaches like using lentiviral vectors (LVV). Permanent therapeutic protein production applications of HSC targeted integration include expression of proteins and enzymes in target organs, including the nervous system (CNS) by tissue resident HSC-derived myeloid cells, as well as efficient systemic delivery of secreted proteins in the circulation. Potential applications include enzyme replacement for metabolic disease, CNS delivery of therapeutic proteins, or antibodies for neurodegenerative diseases, and production of plasma proteins for coagulation and complement disorders. However, given these programs use the same gene editing platform technology as nula-cel, we do not currently intend to continue development of the programs mentioned below.
Our candidates developed to-date harness two genomic locations for targeted insertion, the C-C chemokine receptor type 5 (CCR5) safe harbor locus and the alpha globin locus:
8
We believe there is the potential for NGTC to expand the number of patients and indications addressable with precision engineered, one-time HSC-based treatments and cures. Distinct from our research collaboration with Jasper on JS191, we are evaluating and assessing additional novel conditioning agents and have initiated discovery research efforts to develop antibody-based NGTC candidates.
Our Pipeline
Our Team
Our team is led by executives who have deep experience in drug development and company-building in the biopharmaceutical industry.
Our President and Chief Executive Officer is Josh Lehrer, M.D., formerly the Chief Medical Officer at Global Blood Therapeutics, Inc. (GBT) where he led development for the marketed SCD treatment Oxbryta™ from pre-IND stages through its commercial launch. Prior to GBT, he served in clinical roles at Genentech, Inc. (Genentech) and as a practicing cardiologist at Stanford. Alethia Young is our Chief Financial Officer and has more than 20 years of experience in healthcare and biotech equity research and investing, most recently at Cantor Fitzgerald where she served as senior biotechnology analyst and head of research, managing the equity research department covering companies across the biotechnology industry.
We have a broader leadership team that is passionate about our mission of urgently translating groundbreaking science to transform lives
.
9
Our Strategy
In January 2023, we announced a voluntary pause of our Phase 1/2 CEDAR study of nula-cel for sickle cell disease due to a serious adverse event in the first patient dosed, which we concluded is likely related to study treatment. In February 2023, we announced our decision to discontinue the development of nula-cel and initiate a process to explore strategic alternatives. As a result of this decision, we announced a corporate restructuring that will result in an approximately 50% reduction in workforce. We also disclosed our intention to continue research activities associated with our early-stage non-genotoxic conditioning program, with the goal of advancing toward one or more potential development candidates.
Current Approaches to Gene Therapy and Gene Editing and Their Limitations
Background on Genetic Disorders
A genetic disorder is a disease caused by an abnormal change in a person’s DNA. Most genetic disorders are caused by a mutation in a single gene (monogenic disorder) which results in deficient or defective protein function. These mutations come in many different forms, including:
Mutations that cause genetic disease can either cause loss of function or a toxic gain of function of an important protein. For example, XSCID is caused by lack of functional IL2RG protein, Gaucher is caused by loss-of-function mutations in the GBA1 gene leading to dysfunctional GCase, and cystic fibrosis is caused by the lack of functional CFTR protein. Examples of toxic gain of function, where mutations can cause a protein to have an abnormal and disease-causing function, include SCD where sickle hemoglobin (HgbS), which has a tendency to polymerize in red blood cells, causes damage to the red blood cells, or Huntington’s disease where the huntingtin proteins injure neurons.
Evolution of Genetic Medicines
Genetic medicines have advanced rapidly over the past decade. Initial gene addition approaches have yielded multiple approved products. CRISPR-Cas9 approaches for gene knock-outs are now being translated into the clinic. Base editing builds upon CRISPR-Cas9 and enables targeted editing of certain point mutations.
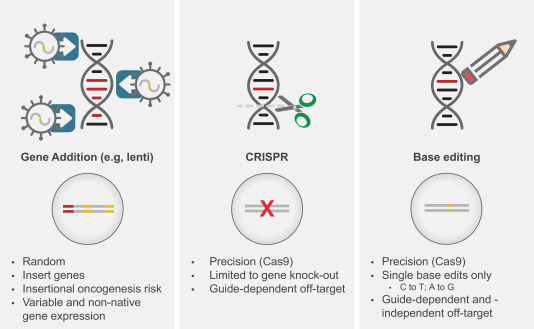
Figure: Evolution of Genetic Medicines
Gene Addition
10
In gene addition, a functional copy of a normal gene is introduced into a cell, typically by a non-integrating viral vector, to drive expression of a normal protein. Recently approved therapies use this approach for spinal muscular atrophy and mutation-associated retinal dystrophy. Other approaches use viral vectors, such as retroviruses and LVV, which randomly integrate a therapeutic gene into the genome for permanent expression.
The principal limitations of gene addition approaches are:
Gene Editing
Gene editing approaches using CRISPR-Cas9 or similar CRISPR nuclease-based technologies are in, or will shortly be initiating, clinical development. CRISPR-Cas9 creates double-stranded breaks in DNA which can be repaired in two primary ways: 1) non-homologous end joining (NHEJ) which creates targeted insertions or deletions (INDELs) or 2) HDR, which can precisely replace DNA at the target cut site by copying from a template. When CRISPR was first shown to be a gene editing tool in human cells, the primary goal and most powerful anticipated application was to use CRISPR with HDR to allow precise gene correction, replacement and insertion. However, repair following CRISPR overwhelmingly favors NHEJ, and due to technical challenges and limitations, efficient use of HDR was not possible in human cells. For this reason, current CRISPR nuclease-based technology is being developed using NHEJ to create INDELs that cannot repair genes, but can alter gene expression. Because RNA guides are used to target Cas9 enzyme (or other CRISPR nucleases) to specific DNA sites, gene editing has much higher precision than earlier methods of permanently modifying the genome, such as gene addition by viral vector integration, and reduces the theoretical risks of insertional oncogenesis with these methods.
CRISPR-Cas9 mediated INDEL (insertions or deletion of bases in an organism’s genome) formation is well suited to introducing new mutations that can disrupt and knock out a target gene. Because the vast majority of genetic diseases are caused by a mutation resulting in loss of function of an important protein, CRISPR INDEL approaches to potentially cure genetic diseases generally require an indirect approach to treat disease and are not able to directly correct the disease-causing mutation. For instance, in SCD, emerging approaches in preclinical and clinical development attempt to knock out Bcl11a function in order to induce fetal hemoglobin expression, rather than directly correcting the point mutation in the HBB gene that causes SCD. Three programs using CRISPR INDEL approaches are currently in clinical development, of which one program has provided initial clinical validation for the safety and potential efficacy of using such approaches for autologous cell therapies.
The principal limitations of gene editing using CRISPR-Cas9 are:
Base Editing
Base editing harnesses CRISPR-Cas9 to deliver a deaminase to a target DNA site, resulting in making a single nucleotide change in the target DNA. This is potentially an advance over nuclease only approaches because it allows direct targeting of a subset of mutations that cause genetic disease. To our knowledge, no base editors have entered clinical development.
11
The principal limitations of base editing are:
Our Next-Generation Gene Editing Approach
Our approach builds on the precision and clinical validation for current gene editing approaches to achieve an entirely new outcome—high-efficiency targeted gene integration. This has the potential to expand the therapeutic opportunities for gene editing beyond conventional gene editing and base editing to enable efficient correction of any type of disease-causing genetic lesion. Beyond gene correction and replacement, this approach is designed to allow the insertion of new therapeutic genes into cells with significantly greater precision and efficiency than existing approaches. We believe this enables broad therapeutic applications ranging from correcting mutations, engineering cells to permanently deliver therapeutic proteins, and precisely engineering effector cells to treat or cure a wide range of serious genetic and other diseases, including cancer, autoimmune and neurodegenerative diseases.
Our innovative approach is a new platform technology built using our deep stem cell biology experience and proven CRISPR technology to efficiently harness a high-fidelity DNA repair process called HDR to integrate DNA copied from a DNA template into genes. Our approach can be described as “find & replace.” We employ CRISPR technology to find and cut a target gene and harness HDR to “copy and paste” replacement DNA from a template. We have demonstrated high-efficiency targeted gene integration across numerous cell types and curative potential in multiple animal models.
Our next generation gene editing technology was designed to create a precise incision in a target gene using a modified, high fidelity CRISPR-based nuclease and we then induce conditions in target cells that overwhelmingly favor DNA repair by a mechanism that relies on HDR rather than the less desirable and more error-prone repair mechanism known as non-homologous end joining or NHEJ. HDR repairs DNA using a DNA template and results in high fidelity copying of template DNA into the correction site while reducing the introduction of DNA mutations that occur with first generation NHEJ gene editing approaches. We achieve HDR-mediated repair by using a non-integrating AAV6 viral vector to deliver template DNA (also called donor DNA) to the target gene. The donor DNA contains 400 base pair DNA segments homologous to sequences (homology arms) on either side of the targeted DNA break, and up to 4 kb of new DNA sequences between these homology arms. The cell’s natural DNA repair process uses the homology arms to align the template in the correct location, and then copies and pastes the new DNA into the genome at the targeted gene cleavage site. This process enables correction or replacement of a mutated gene, or insertion of a new therapeutic gene in a precise location.
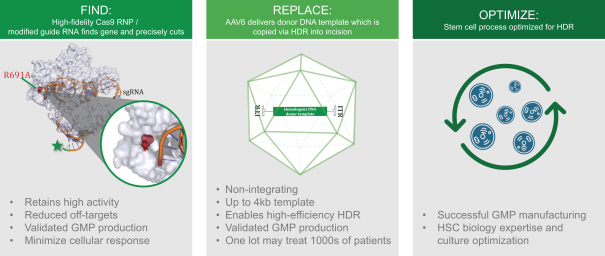
High Precision CRISPR-Based Nuclease
Our founders discovered that chemically modified guide RNAs can enhance Cas9 activity and subsequently showed that delivering Cas9 as a recombinant protein instead of as mRNA further increased cutting efficiency. These approaches are now widely used and widely considered to be state of the art for gene editing. We have continued to optimize the CRISPR component of our platform and employ an improved Cas9 enzyme with dramatically reduced off-target activity in preclinical models. We employ high fidelity (HiFi)
12
Cas9, which was co-discovered by our founders and for which we have exclusively licensed patent rights from Integrated DNA Technologies, Inc. (IDT) in certain fields, to reduce off target cutting by 20-fold on average and 30-fold on average for the SCD gene in preclinical models, thus providing potential improved safety. We believe this is a unique advantage for our programs.
Harnessing HDR
Cells naturally have the ability to repair their DNA if damaged. One highly specialized repair pathway is called HDR because the cell uses a homologous template to precisely “copy and paste” DNA sequences to repair a DNA break without introducing errors. Normally, the template used in HDR comes from the sister chromosome. Because of its precision and ability to use a template, harnessing the HDR pathway to achieve therapeutic targeted gene integration has been a long-sought but elusive goal due to its potential to dramatically expand gene editing’s applications and curative potential.

To achieve “find & replace,” as described above, we deliver an optimized, synthetic DNA template via a non-integrating AAV6 viral vector which is transduced into cells. Our founders evaluated various approaches before discovering that AAV6 achieved the most efficient transduction in comparison to nine other AAV serotypes, while optimally preserving stem cell function. Our AAV6 donor DNA template was iteratively optimized to maximize the efficiency of targeted gene integration. No viral genes are present in the template, and the template itself exists only transiently in the target cell population.
Process Optimization
HDR is most active during cell division and is inefficient in slowly dividing cells like HSCs. Achieving HDR at potentially curative efficiency in HSCs has been an elusive and highly sought goal because HSCs are long-term multi-potent stem cells with broad therapeutic potential and potential lifetime durability. In our process, we use clinically validated and standard methods to isolate HSPCs from patients, which are comprised of both slowly dividing HSCs (lower rates of HDR) and more rapidly dividing progenitors (higher rates of HDR). Although edited HSPCs are the standard drug product for any gene edited autologous stem cell therapy, the therapeutic effect comes from the long-term HSCs that are a subset of the cells in the drug product. Harnessing our stem cell biology expertise, we optimized the timing of template delivery and cell culture conditions to improve gene correction frequency from approximately 20% in initial experiments to approximately 70% in human HSPCs in nula-cel, our sickle cell program. We believe this gene correction rate in
13
HSPCs ensures that the correction rate in the long-term stem cells can achieve the threshold required to cure patients (estimated to be engraftment of 20% corrected cells).
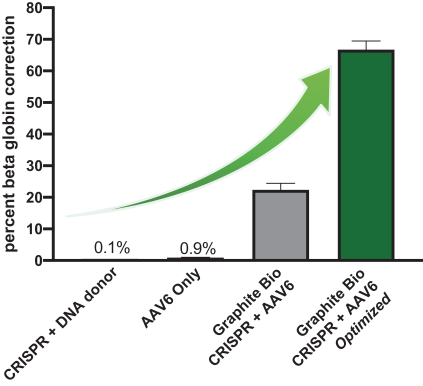
We believe our technology platform is revolutionary because it brings together proven individual technologies, new discoveries, and systematic process optimization to, for the first time, achieve HDR-mediated targeted gene integration at efficiencies of up to approximately 70% in human HSPCs in ex vivo studies. We have serially optimized our GMP process to retain high and potentially curative gene correction rates at clinical scale.
Our approach differs from first generation gene and base editing technologies:
14
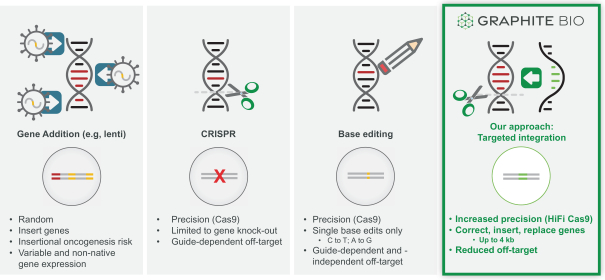
Key Differentiated Components of Our Technology Platform
Our platform combines two powerful, well-characterized biologic approaches—CRISPR and HDR—with our HSC expertise and know-how to achieve high-efficiency targeted gene integration.
Efficient cutting with a CRISPR-based nuclease is an important first step in our process. Our founders discovered that chemically modified guide RNAs can enhance Cas9 activity and subsequently showed that delivering Cas9 as a recombinant protein instead of as mRNA further increased cutting efficiency. These approaches are now widely used and widely considered to be state of the art for gene editing. We have continued to optimize the CRISPR component of our platform as described below together with additional differentiated and proprietary components in our technology and process:
15

Figure: HiFi Cas9 had an approximately thirty-fold reduction in off-target DNA cleavage compared to wild-type Cas9.
To determine relative transduction efficiencies across AAV serotypes, human primary hematopoietic progenitors were infected with ten AAV serotypes each carrying the green fluorescence protein (GFP) reporter gene. The experiment was designed to determine relative transduction efficiency rather than to maximize transduction. As shown in the figure below, we observed that AAV6 was most efficient in comparison to nine other AAV serotypes. Our founders later discovered that additional optimization and ribonucleoprotein (RNP) electroporation prior to AAV6 transduction further enhanced AAV transduction efficiency.
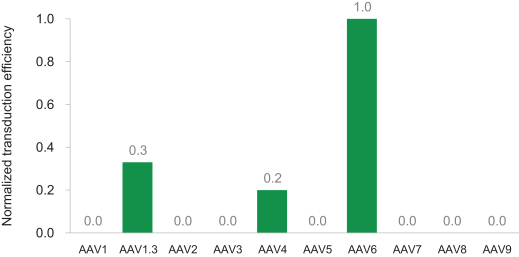
Figure: AAV transduction of human primary hematopoietic progenitor cells.
16
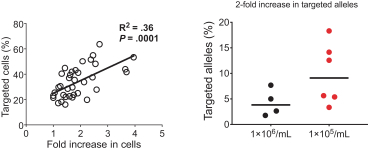
Figure: Optimization of HSC cell culture conditions led to an increase in the rate of homologous repair and gene insertion.
We have found that each of these optimization steps and our other know-how can contribute to the creation of a highly efficient targeted gene integration process. We have further optimized our process to maintain high levels of efficiency at clinical scale using HSPCs isolated from healthy donors.
Expanding Eligible Patients and Potential Indications: Combining Our High-Efficiency Approach with Advances in Non-Genotoxic HSC Targeted Conditioning (NGTC)
One of our key strategic priorities is to make our potential one-time curative HSC-based therapies available, if approved, to as many appropriate patients as possible. We intend to do this by harnessing industry and internal research advances in non-genotoxic HSC targeted conditioning (NGTC) regimens. We believe the high precision and high efficiency with which we can consistently introduce genes by HDR has the potential to greatly expand the application to treat more patients and address more types of diseases for which gene editing-based therapies are feasible.
A limitation of therapies based on ex vivo genetic manipulation of HSCs is that the patient must be pre-conditioned with non-targeted, genotoxic conditioning agents, to both eliminate the dysfunctional endogenous HSCs and to create room for the modified cells to engraft and expand. This approach is standard for allogeneic bone marrow transplant (e.g., for SCD) and for approved HSC gene therapy products and has safety risks such as transient neutropenia, which necessitates prolonged hospitalization, potential fertility impairment, and the risk of secondary malignancies. These risks may reserve use of ex vivo HSC-based genetic and potentially curative therapies for diseases with limited treatment options, and for the most severely affected patients.
Applications Enabled by Our Technology
We believe our platform can be applied in the following three key settings: gene correction, gene replacement and targeted gene insertion.
17
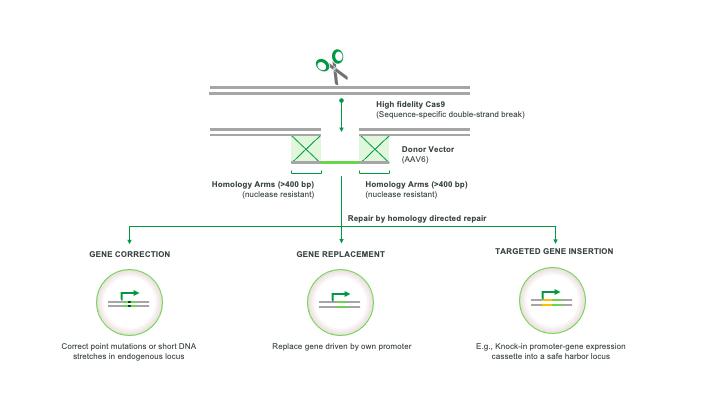
Our Product Candidates
Gene Correction: nulabeglogene autogedtemcel (nula-cel) for the Treatment of SCD
Overview of nula-cel
In January 2023, we announced a voluntary pause of our Phase 1/2 CEDAR study of our lead product candidate nula-cel due to a serious adverse event in the first patient dosed, which we concluded is likely related to study treatment. In February 2023, we announced our decision to discontinue the development of nula-cel and initiate a process to explore strategic alternatives.
Nula-cel is a next generation gene-edited autologous HSC product candidate that is designed to directly correct the mutation responsible for SCD. The mortality and morbidity associated with SCD, all caused by a single mutation, has made curing SCD by direct gene correction a dream of many clinicians. Indeed, multiple genetic therapies are in development to address SCD, but due to technical limitations of other approaches, these therapies are primarily focused on expressing alternate hemoglobin genes such as fetal hemoglobin or a transgenic hemoglobin. Our approach is the first in industry to directly correct the SCD-causing mutation to restore normal adult hemoglobin expression
.
Overview of Sickle Cell Disease
SCD is caused by a single nucleotide substitution in the gene encoding the beta subunit of hemoglobin (Hb), resulting in the production of sickle hemoglobin (HgbS). SCD is an autosomal recessive disease, meaning individuals with SCD have two copies of the mutated beta-globin gene. HgbS polymerizes in red blood cells to form rigid rod-like structures, damaging cell membranes and causing red blood cells to take on a characteristic sickle shape ultimately resulting in hemolytic anemia (destruction of red blood cells) and vaso-occlusion (blockages in blood vessels), the two major pathophysiologic features of SCD. The anemia and vaso-occlusion cause severe symptoms, serious morbidity including multiple organ damage, and shortened lifespan.
SCD is the most common monogenic blood disorder with an estimated global incidence of over 300,000 births annually. Population estimates suggest that there are approximately 100,000 persons living with SCD in the United States with an additional 67,000 people living with the disease in the European Union. The global prevalence of the disease is estimated to be about 20-25 million. Unaffected biological parents of individuals with SCD have sickle cell trait. Sickle cell trait is the benign carrier status (one copy of normal and one copy of mutated beta-globin) of SCD present in over 100 million people worldwide.
SCD is a serious and life-threatening disease. Quality of life is often poor and life expectancy is reduced by 20-30 years. Patients experience severe, often daily symptoms of pain and fatigue, suffer from acute painful episodes often requiring hospitalization, and are at risk for serious complications and organ damage including stroke, silent cerebral infarction, osteonecrosis, renal failure, pulmonary hypertension and cardiomyopathy.
Sickle Cell Disease—Available Treatments and Unmet Needs
There are four available therapies approved by the FDA for SCD treatment: hydroxyurea, L-glutamine, and Adakveo™ (crizanlizumab) to reduce the frequency of vaso-occlusive crises (VOCs), and Oxbryta™ (voxelotor) to increase hemoglobin levels and reduce hemolysis. These therapies require lifelong usage and may in some cases reduce but do not eliminate SCD’s serious symptoms or complications. None of these therapies have been shown to prevent pain or organ damage, or to increase survival. Chronic blood
18
transfusion therapy is another treatment option for some SCD patients. While transfusion therapy has a role in decreasing risk of stroke, a dreaded SCD complication, it has significant side effects including iron overload. Despite advancements in current care, progressive organ damage continues to cause early mortality and severe morbidity.
Allo-HSCT remains the only curative therapy for SCD and is considered the gold-standard for potentially curative therapies. The HSCT procedure ablates the patient’s endogenous HSCs that produce sickle red blood cells and replaces them with normal HSCs, typically from a matched sibling donor with sickle trait. HSCT is considered curative because donor cells contain at least one corrected copy of the beta-globin gene and produce normal adult HgbA yielding normal red blood cells, thereby preventing disease complications. HSCT with donor sickle trait cells has been shown to be curative because every red blood cell contains approximately 55% HgbA protein and 45% HgbS protein and does not sickle. In patients who have been cured by HSCT, this results in the elimination of VOCs and prevention of the progressive organ damage that leads to shortened lifespan. HSCT is the only therapy for SCD proven to have an impact on VOCs, organ damage and mortality. However, HSCT is rarely used due to the difficulty in finding a matched donor (as low as 16-19%), safety risks, including graft-versus-host-disease, and need for long-term immunosuppression.
Despite HSCT’s limitations (including approximately 90% of African Americans not having an available matched sibling donor, and the need for life-long immunosuppression), more than 150 HSCT procedures are performed in the United States annually. We believe this indicates substantial underlying demand for curative options, which is driven by SCD’s severity and the limitations of other current treatment options.
Sickle Cell Disease—Emerging Curative Treatments and Potential Limitations
Gene therapy and gene editing approaches are attractive alternatives to HSCT because a patient’s own cells (autologous cells) are genetically modified and therefore do not face the high risk of rejection or graft-versus-host disease associated with allo-HSCTs. However, it is unclear whether gene therapy (gene addition) and gene editing (HgbF induction) approaches currently in the clinic can achieve long term benefits similar to allo-HSCT, which directly replaces stem cells with HbSS genotype with normal (HbAA) or sickle trait (HbAS) stem cells from a matched sibling donor.
Gene addition approaches coopt a LVV to semi-randomly integrate a modified gene for non-sickling beta (or gamma) hemoglobin into the genome, leaving the disease-causing sickle globin gene intact. Results from these trials are promising and demonstrate that patients treated using this approach have reduced VOC incidence, significant hemoglobin increases and reduction in hemolysis. However, the random insertion of newly introduced genes raises safety concerns for a potential increased risk of tumorigenesis. Use of viruses such as LVV to insert genes also results in a high variability in the number of gene copies that are inserted into the genome. This leads to variable expression levels of transgenic hemoglobin such that a significant proportion of red blood cells may not be protected. Finally, LVVs have a biologic preference for integrating into the introns of actively expressed genes, which could cause long-term perturbations of HSC function that may take years to manifest themselves.
A different, yet indirect, approach uses CRISPR-Cas9 gene editing to reduce or eliminate the suppression of HgbF expression, thereby increasing HgbF levels. As with LVV gene addition, this approach also leaves the disease-causing sickle mutation intact. The rationale for this approach is that rare patients with naturally occurring elevated HgbF levels may have reduced or minimal SCD symptoms. Data available on three treated patients suggests that this HgbF induction also reduces the rate of VOCs and results in significant hemoglobin increases and reduction in hemolysis. HgbF serves to transfer oxygen from the maternal blood stream to the fetus because it has a higher oxygen affinity compared to adult hemoglobin. HgbF is normally expressed only in the fetus and replaced by HgbA within one year of birth. Due to its abnormally elevated oxygen affinity for adults, prolonged elevated HgbF expression may result in adverse physiological consequences. Additionally, HgbF has not been shown to have an impact on end organ damage, which is the main cause of early mortality in patients with SCD.
Therefore, we believe that current gene editing and gene addition approaches, while promising, stop short of correcting the underlying disease-causing mutation and addressing all of the complications associated with the disease, which remains the ultimate goal of a curative therapy for SCD.
Nula-cel
Although we have discontinued the development of nula-cel in SCD, nula-cel was the first targeted genetic therapy in clinical development designed to efficiently and precisely correct the disease-causing gene, simultaneously eliminating HgbS production and restoring normal HgbA expression. At the DNA level, we believe this is the first approach in the industry that seeks to convert an SCD genotype (two genes with sickle mutations, HbSS) to a normal genotype (at least one normal beta-globin gene). By correcting the SCD-causing mutation, our next-generation gene editing approach has the potential to overcome a major limitation of current gene addition
19
and gene editing approaches that take an indirect approach. Our goal with nula-cel is to replace a sufficient quantity of a patient’s HSCs with gene corrected cells to definitively cure SCD.
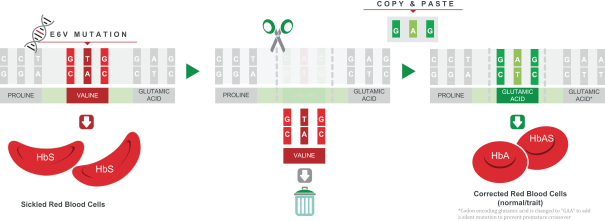
Figure: nula-cel removes the mutated region of HbS and replaces it with that of a normal hemoglobin gene.
In order for this approach to be curative in patients, it is not necessary to correct all sickle globin genes nor to correct all HSPCs. Because sickle cell trait individuals have benign SCD carrier status, correcting one out of the two sickle globin genes in a cell is sufficient to correct that cell. Furthermore, to cure the disease, it is not necessary to correct all SCD HSPCs. In patients who received allo-HSCT from a matched sibling donor with sickle trait—long-term, persistent mixed donor chimerism where only 20% of HSCs have normal hemoglobin resulted in cures, and clinical benefits were observed with as low as 5% corrected cells. Per the figure below, we have shown under IND-enabling GMP manufacturing conditions that we can achieve correction (meaning one or more corrected copies of the sickle globin gene) in over 55% of treated HSPCs, which we believe to be well above the predicted curative threshold.
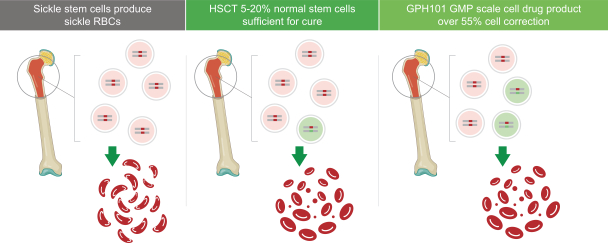
Figure: We have shown that under IND-enabling GMP manufacturing conditions, we can achieve HbS gene correction above the predicted threshold required for cure.
We believe that nula-cel has the potential to be the optimal curative approach, because it is designed to directly correct the mutation responsible for SCD and restore normal biology by eliminating HgbS production and restoring HgbA expression. Based on existing allo-HSCT data, this supports nula-cel’s potential to address the complications associated with the disease and provide a definitive cure.
Preclinical Data
We have used both healthy donor and sickle cell disease patient-derived hematopoietic stem cells in our preclinical studies. Although correction of the sickle mutation requires sickle hematopoietic stem cells, we can also perform the same process on cells from healthy donors because the DNA template introduces additional silent (no change to amino acid coding sequence) nucleotide changes by HDR. Overall, our data highlight that HBB (beta-globin) gene correction is equivalent in healthy donor as well as sickle cell disease patient-derived hematopoietic stem cells.
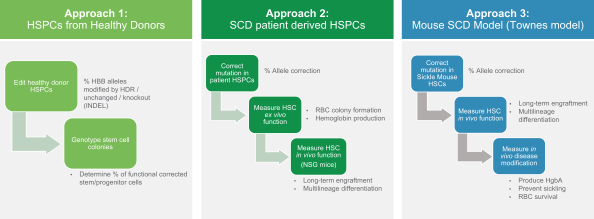
20
We have taken three experimental approaches to generate preclinical proof of concept data for nula-cel. The first approach evaluates HDR efficiency in HSPCs from healthy donors subsequently measuring both the frequencies of HBB allele editing in the bulk population and edited cell HBB genotypes (e.g., the percentage of cells with at least one corrected allele). The second approach corrects the HbS gene in HSPCs isolated from patients, then measures the function of treated cells both ex vivo and in a humanized mouse model. The third approach corrects the HbS mutation in HSCs from a sickle mouse model and assesses corrected cells’ ability to modify the disease.
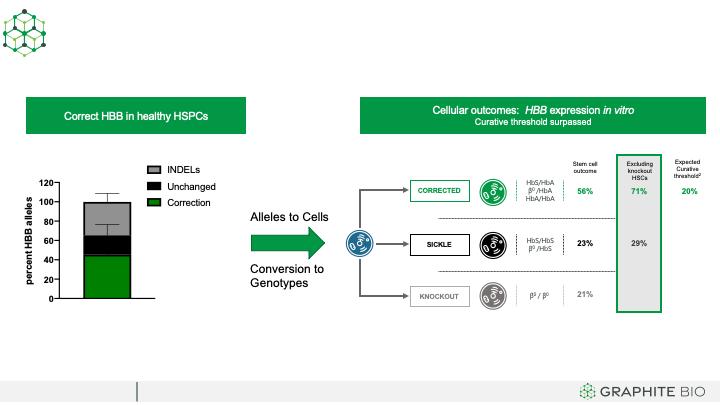
In experimental approach 1, illustrated in the left panel of the figure below, HSPCs were isolated from healthy donors. We then used CRISPR to target HBB alleles and then introduced silent mutations by HDR from the AAV6-delivered donor DNA template, a process equivalent to the intended process for clinical samples. In these healthy donor cells, HDR modified HBB alleles are equivalent to corrected alleles, and unchanged alleles are equivalent to sickle alleles. Over 40% of HBB alleles were corrected, approximately 40% had INDELs, and approximately 20% of HBB alleles remained unchanged. We anticipate from this experiment that creating INDELs in the HbS gene may be beneficial to SCD patients because INDELs may prevent sickle hemoglobin expression through knockout of the HbS gene, and stem cells containing biallelic sickle globin INDELs will not be able to produce sickle RBCs. To understand the impact of corrected and INDEL alleles on stem and progenitor cell genotype, and on the probability of achieving the predicted curative threshold of 20% corrected cells, we next genotyped individual stem and progenitor cell colonies. Results are shown in the right panel of the figure below. We observed that 40% of corrected alleles translated into 56% of stem cells being the equivalent of corrected (monoallelic or biallelic HDR), 23% are equivalent of sickle (unchanged), and 21% are knockout (INDEL/INDEL). Because knockout stem cells do not make functional RBCs, the proportion of functional corrected stem cells is approximately 70% (56% corrected colonies divided by 79% colonies that can make normal adult hemoglobin), which is well above the expected curative threshold of 20%. Thus, our approach of both knocking out the disease-causing mutation and applying subsequent gene correction to restore the HbA gene has the potential to lead to higher than anticipated cell correction rates and increases our confidence in the ability to exceed the expected curative threshold.
SCD Patient Derived HSPCs
In experimental approach 2, HSPCs were isolated from SCD patients and edited utilizing a process similar to the intended process for clinical samples, as illustrated below in the left panel of figure below. Due to our optimized process, over 60% of HbS alleles were
21
corrected, approximately 20% had INDELs and only approximately 10% of HbS alleles remained intact. We believe that the INDELs may be beneficial to SCD patients since INDELs prevent expression of sickle hemoglobin from the uncorrected intact HbS genes.
Next, these edited HSPCs were differentiated into red blood cells ex vivo and their hemoglobin expression was measured. As illustrated in the middle panel of the figure below, analysis of HgbA and HgbS expression (subtracting background HgbF levels) showed over 90% normal hemoglobin A and only approximately 10% sickle hemoglobin. We believe this result was better than expected for sickle trait, where red blood cells contain 55% HgbA protein and 45% HgbS protein, because INDEL formation in uncorrected sickle alleles eliminated most HgbS expression. As illustrated in the right panel of the figure below, when transplanted into immunodepleted NSG mice, these cells engrafted in a long-term (16 weeks), stable fashion with approximately 30% of sickle alleles corrected. This translates into approximately 40% of the long-term HSCs being corrected by containing at least one corrected sickle allele, double the expected curative threshold in humans. We can measure corrected alleles more directly than corrected cells; the curative threshold based on corrected alleles is anticipated to be approximately 15% because the percent of cells that have at least one corrected allele is approximately 1.3 times higher than the percent of corrected alleles. Possible reasons for the approximately two-fold difference in gene correction between the infused HSPCs (approximately 70%) and the HSCs engrafted in the mice (approximately 35%) include that long term engrafting HSCs have lower efficiency HDR than progenitor cells that comprise the majority of HSPCs; that this is a feature specific to the mouse model; or that the gene correction process impairs functionality of some of the HSCs. Regardless of the explanation, the 30% gene correction seen in vivo in engrafting HSCs is predicted to be curative in humans.

Figure: nula-cel created from SCD patients predominantly expressed normal hemoglobin and led to stable engraftment in immunodeficient mice
Mouse SCD Model (Townes Model)
The Townes model of SCD is a transgenic mouse model in which the mouse hemoglobin locus is replaced with human HbA and HbS genes. These mice express sickle cell hemoglobin and exhibit many of the symptoms of human SCD including red blood cell sickling and short red blood cell half-life. In this experimental approach HSCs were isolated from sickle mice and edited utilizing the same process as the process for human HSPCs. As illustrated in the left panel of the figure below, we observed that approximately 20% of sickle alleles were corrected in the mouse HSCs, likely because of processes that were optimized for human and not mouse HSCs. Given the estimated curative threshold in humans of 20% HSCs, we predicted that mice achieving 20% or greater correction of engrafted cells (15% of alleles) would show substantial benefit of disease features. As illustrated in the center panel of the figure below, all mice with greater than 15% allele correction showed a profile of hemoglobin expression consistent with a potential cure with over 55% HgbA protein (same HgbA level as sickle trait). Furthermore, red blood cells from gene-corrected mice had a half-life that was approximately
22
ten-fold longer than SCD mice. As illustrated in the right panel of the figure below, we observed that these gene-corrected red blood cells were resistant to sickling.
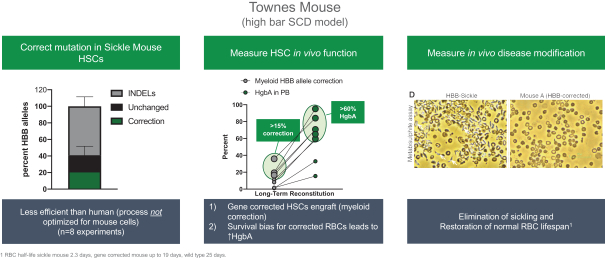
Figure: Gene correction in a humanized SCD mouse model resulted in over 70% normal hemoglobin expression leading to reduced red blood cell sickling.
Nula-cel Phase 1/2 Clinical Trial
In November 2021, we initiated a Phase 1/2 open label clinical trial of nula-cel in approximately 15 patients with severe SCD. The primary objective of this trial was to assess safety. Secondary objectives of the trial were to evaluate engraftment success, gene correction rates, total hemoglobin, hemoglobin A and S, effect on clinical events such as VOCs and exploratory endpoints that could generate initial evidence of nula-cel’s ability to restore red blood cell health and have an impact on end organ damage. In January 2023, we announced a voluntary pause of our Phase 1/2 CEDAR study of nula-cel for SCD due to a serious adverse event in the first patient dosed, which we concluded is likely related to study treatment. In February 2023, we announced our decision to discontinue the development of nula-cel and initiate a process to explore strategic alternatives.
Gene Replacement: GPH102 for the Treatment of Beta-Thalassemia and GPH201 for the Treatment of XSCID
GPH102 for the Treatment of Beta-Thalassemia
Given this program leverages the same gene editing platform technology as nula-cel, we do not currently intend to continue development of this program.
Beta-thalassemia is a genetic disorder characterized by reduced production of beta-globin, a protein that forms functional, oxygen-carrying hemoglobin with alpha-globin (HbA, a2b2). In its most severe form, beta-thalassemia is caused by mutations in both alleles of the HBB gene. These patients fail to produce functional beta-globin, resulting in severe anemia. More than 300 beta-thalassemia mutations are known; most are small nucleotide insertions, substitutions, or deletions within or directly adjacent to the HBB gene.
The most common treatment for beta-thalassemia is chronic blood transfusions. Transfusion-dependent patients typically receive transfusions every two to four weeks. Chronic administration of blood often leads to elevated levels of iron in the body, which can cause organ damage over a relatively short period of time. Patients are often given iron chelators, or medicines to reduce iron levels in the blood, which are associated with their own significant toxicities. In developing countries, where chronic transfusions are not available, most patients die in early childhood. A treatment for anemia in adult patients with transfusion-dependent beta-thalassemia, Reblozyl (luspatercept-aamt), received FDA approval in 2019. While Reblozyl significantly reduced transfusion burden, it did not eliminate the need for transfusions. The only known curative therapy for beta-thalassemia is allo-HSCT, but utilization is limited by lack of matched and willing donors and need for lifelong immunosuppression. As with SCD, evidence suggests that allo-HSCT can be curative if 20% or more normal donor cells engraft.
GPH102 leverages our platform, replacing the HBB gene via HDR to achieve a normal or beta-thalassemia trait genotype with the goal of restoring HgbA expression to levels similar to healthy individuals. Alternative genetic therapies are in clinical development to address beta-thalassemia, but in general due to technical limitations, these potential therapies are indirect and are focused on expressing alternate hemoglobin genes such as fetal hemoglobin (HgbF) or a transgenic hemoglobin without correcting the underlying genetic lesions. The principal limitations of HgbF upregulation are currently understood to be that (1) its abnormally elevated oxygen affinity may result in adverse physiological consequences in adults and (2) individual RBCs require sufficient levels of beta or beta-like
23
globin to prevent hemolysis. It is currently unclear whether indirect approaches such as HgbF upregulation can achieve this, especially in the most severe b0/b0 genotype. The principal limitations of transgenic hemoglobin addition approaches are currently understood to be that: (1) the LVV that delivers the transgenic gene may have a biologic preference for integrating into the introns of actively expressed genes, which could cause long-term perturbations of HSC function that may take years to manifest themselves and (2) transgenic beta-globin levels are variable between RBCs (due to variable vector copy number and integration site). Additionally, some RBCs may express insufficient transgenic beta-globin to be protective, especially in the most severe b0/b0 genotype.
GPH201 for the Treatment of XSCID
Given this program leverages the same gene editing platform technology as nula-cel, we do not currently intend to continue development of this program.
GPH201 is an investigational next generation gene-edited autologous HSC product candidate for the treatment of XSCID. XSCID is a rare, life-threatening disease where multiple mutations in a single gene prevent the formation of multiple interleukin receptors resulting in defects in immune cell formation. As a consequence, severe, persistent, or recurrent early-onset infections are the hallmark of XSCID. Without treatment, infants with XSCID usually do not live beyond one year of age. Allogeneic HSCT that results in functional reconstitution of the immune system is the only curative treatment for XSCID, but the procedure has limitations including identification of an HLA matched sibling donor as well as potential complications of GvHD and subsequent poor immune reconstitution. An effective targeted genetic therapy would need to replace a large portion of the IL2RG gene in order to be effective across XSCID patients with different IL2RG mutations. The goal of GPH201 is to replace a sufficient quantity of a patient’s HSCs with gene edited cells to eliminate the symptoms of, and potentially cure, XSCID.
In preclinical studies assessing GPH201’s gene replacement efficiency, we modified HSCs from healthy males using our GPH201 process. As illustrated in the figures below, we observed an overall mean IL2RG gene replacement efficiency of approximately 45% in healthy donor-derived HSPCs. These HSCs were then engrafted in bone marrow of immunodeficient mice, where approximately 30% gene replacement was observed, indicative of long-term curative potential. We believe this level of gene replacement is well in excess of the estimated 1-5% curative threshold.

Figure: The IL2RG gene was replaced in approximately 45% of treated HSPCs from healthy donors
To assess the potential of GPH201 to restore the ability of progenitor cells to differentiate into T cells and NK cells, we isolated HSPCs from an XSCID patient with subsequent replacement of the IL2RG gene, achieving approximately 40% gene replacement efficiency, as illustrated in the figure at left below. Upon differentiating these cells in vitro, as illustrated in the figure at right below, treated HSPCs from the XSCID patient had an approximately nine-fold increase in cells that formed T cells, B cells, and NK cells than untreated control cells.
24

Figure: IL2RG gene replacement in XSCID patient HSPCs led to significant increase in T cell and NK cell formation
As a result of the selective advantage of progenitor and effector cells that express normal IL2RG, it is estimated that only 1-5% of genetically corrected HSCs would be needed to reconstitute immunity in XSCID patients. This selective advantage is highlighted by reports that rare XSCID patients have had a somatic reversion in a single precursor cell that led to reconstitution of their immune system for years. Based on the editing efficiency we have demonstrated and the low number of genetically corrected HSCs needed to potentially cure the disease, we believe that GPH201 can be curative and could be combined with a novel, safer and targeted bone marrow conditioning approach.
We have partnered with Jasper to assess GPH201 combined with targeted conditioning using JSP191, an anti-CD117 monoclonal antibody and without the use of chemotherapeutic myeloablation. Clinical data has shown that JSP191 has the potential to lead to successful engraftment in allo-HSCT for XSCID. Although XSCID is an ultra-orphan indication with a small number of severely affected patients, we believe GPH201 could address an important unmet need, and data from this program (potentially in combination with JSP191) could be informative to our platform and pipeline.
Targeted Gene Insertion with Therapeutic Protein Production (CCR5 Safe Harbor Locus): GPH301 for the Treatment of Gaucher Disease
Given this program leverages the same gene editing platform technology as nula-cel, we do not currently intend to continue development of this program.
Our GPH301 product candidate is a next generation gene-edited autologous HSC product candidate from our CCR5 locus technology for the treatment of Gaucher disease, an autosomal recessive genetic disorder caused by mutations in the GBA gene which encodes GCase. GCase is an enzyme responsible for degrading glucocerebroside, a cell membrane building block, into glucose and lipids within lysosomes of cells. In patients with Gaucher disease, lack of GCase leads to accumulation of glucocerebroside in macrophages resulting in inflammation that impacts the liver, spleen and bone marrow.
Gaucher disease is the most common inherited lysosomal storage disease and is classified into three types: Type 1 disease is associated with hematologic abnormalities, enlargement of the liver and spleen and skeletal defects, which severely impact quality of life; Type 2 disease causes life-threatening neurological dysfunction in infants, who often die within the first few weeks of life; and Type 3 disease causes severe neurological complications in addition to all the symptoms associated with Type 1 disease, resulting in reduced lifespan. There are approximately 6,000 patients with Gaucher disease in the United States. 90% of Gaucher patients in the United States and Europe are classified as Type 1.
Gaucher disease is currently treated by enzyme replacement therapy (ERT), which is recombinant GCase. All of the approved ERTs are administered as biweekly infusions. Long term ERT for Gaucher disease results in lower levels of anemia, reduced bone pain, and reductions in spleen and liver enlargement, but is not curative. An alternate method of treating Gaucher disease is to block the synthesis of glucocerebroside with inhibitors rather than to accelerate its breakdown with ERT. Approved products in this category
25
include miglustat and eliglustat. These products are not generally as effective as ERT and have significant safety risks. HSCT is the only treatment that can provide a definitive cure for Gaucher disease and is considered prior to the onset of neurologic symptoms.
GPH301 is designed to insert a functional copy of the gene for glucocerebrosidase (GCase) into the chromosomal location of the CCR5 gene. This locus is known as a safe harbor both because of the lack of serious deleterious effects in humans with CCR5 mutations and because the expression of genes inserted there can be precisely controlled by regulatory elements inserted together with the gene of interest. We include the CD68S promoter in the inserted gene cassette which we believe provides two advantages: 1) targeting GCase expression specifically to the disease-causing cell in Gaucher (and avoiding expression in HSCs which could affect stem cell function) and 2) macrophage expression takes advantage of the ability of gene corrected macrophages to cross the blood brain barrier and address the neuropathic manifestation of Type 3 Gaucher Disease. The goal of GPH301 is to replace a sufficient quantity of a patient’s HSCs with gene edited cells to drive GCase expression in a patient’s macrophages and reverse the accumulation of unprocessed glucocerebroside. Data from Gaucher patients with mixed donor chimerism and from mouse models support that less than 10% corrected HSCs could be curative.
In preclinical studies assessing the efficiency of our targeted gene insertion process, we isolated HSCs from healthy donors using our GPH301 process. As illustrated in the left panel of the figure below, we were able to achieve efficient gene insertion as demonstrated by approximately 35% of the targeted CCR5 alleles containing a GCase insertion. As illustrated in the center panel of the figure below, the edited, engrafted cells contain more than 10% alleles with the insertion, which corresponds to more than 15% of the cells, which is above the predicted threshold (5%-10%) for patients to achieve a cure. As predicted, because of our use of the CD68S promoter, GCase expression was restricted to monocytes and macrophages. As illustrated in the right panel of the figure below, GCase expression was two-fold higher in edited versus unedited healthy donor cells in both in vitro cultures and in cells isolated from a humanized mouse model after engraftment. We believe that this preclinical data strongly supports the curative potential of GPH301.
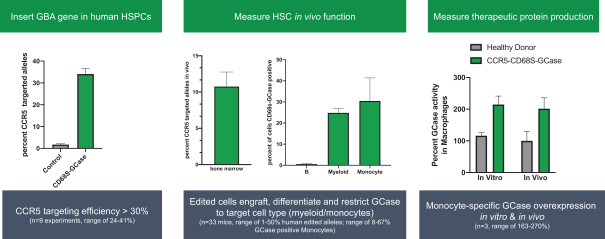
Figure: Insertion of the GBA gene in the CCR5 safe harbor led to the expression of GCase in monocytes.
We believe proof of concept in Gaucher disease could accelerate development of a CCR5 safe harbor protein production pipeline given there are significant synergies and regulatory efficiencies because these programs would use the same RNA guide and preclinical safety assessment.
Future Targeted Gene Insertion with Therapeutic Protein Production (CCR5 Safe Harbor) Opportunities
Given these future opportunities would leverage the same gene editing platform technology as nula-cel, we do not currently intend to pursue them.
We had previously planned to pursue indications beyond Gaucher disease using our CCR5 locus technology for tissue-based protein expression, including for CNS protein delivery. Inserting different genes into this locus using the same sgRNA, integration site and homology arms could enable rapid expansion into other diseases.
Our founders have published animal or in vitro data using the CCR5 safe harbor approach in several indications, including mucopolysaccharidosis type I (MPS I), a severe metabolic disease characterized by buildup of glycosaminoglycans (GAGs) due to a deficiency of alpha-L iduraonidase (IDUA), an enzyme responsible for degradation of GAGs in lysosomes. Without IDUA, GAGs accumulate in the body leading to developmental delays, enlarged organs, neurologic damage which may lead to cognitive decline, and early death. MPS I is treated primarily by chronically administered ERT, for which CNS efficacy is limited because ERT does not cross
26
the blood-brain barrier. The only curative treatment for MPS I is allo-HSCT which is rarely used because of the lack of matched donors and immune complications.
To assess the efficiency of our targeted gene insertion process, we isolated HSPCs from healthy donors using a process similar to that of GPH301. As illustrated in the left panel of the figure below, we were able to achieve efficient gene insertion as demonstrated by approximately 45% of the targeted CCR5 alleles containing a phosphoglycerate kinase promoter (PGK)-IDUA insertion. While we have not optimized gene insertion for IDUA in engrafted cells, targeted cells successfully engraft in immunodepleted mice, and as illustrated in the center panel of the figure below, the edited, engrafted cells contain more than 5% alleles with the insertion, which corresponds to approximately 7% of cells. To study the IDUA enzyme activity contribution in cells containing the PGK-IDUA gene insertion, a pure population of targeted cells was measured in vitro in macrophages. Approximately 30-fold higher IDUA activity was observed versus healthy donors, as illustrated in the right panel of the figure below. We then transplanted the PGK-IDUA human HSPCs into IDUA knockout mice (an MPS I animal model) to assess in vivo IDUA activity. The serum IDUA activity in the transplanted mice was an average of approximately 3% of normal activity versus 0.05% activity in IDUA knockout mice (0.8% activity or higher is expected for clinical benefit based on patients with mild disease). Overall, these data highlight that the CCR5 safe harbor locus is a modular therapeutic protein production platform that has broad applicability for treating genetic diseases, including other lysosomal storage disorders.
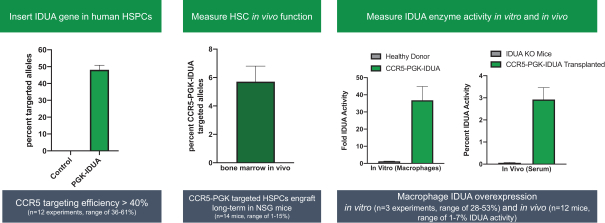
Future Opportunities in Targeted Gene Insertion with Therapeutic Protein Production: Alpha Globin Locus
Given these future opportunities would leverage the same gene editing platform technology as nula-cel, we do not currently intend to pursue them.
For certain therapeutic applications, we believe there is an advantage to precisely inserting a gene into a location in the chromosome where we can utilize a native cell promoter that can lead to high level and lineage specific expression. One such locus is the alpha globin (HBA1) locus, in which the endogenous alpha-globin promoter can be used to express inserted genes in red blood cells or red blood cell precursors to drive therapeutic protein production. This is an attractive approach because the very high rate of red blood cell formation (200 billion produced each day), coupled with the strength of the alpha globin promoter (280 million hemoglobin molecules per red blood cell) could allow for production of normal levels of therapeutic protein with modest HSC engraftment targets (<10%) which may be achievable with non-genotoxic HSC targeted bone marrow conditioning regimens. We believe this could dramatically improve the benefit risk profile of product candidates as potential one-time HSC cures.
A number of blood diseases could potentially be cured or treated by one-time infusion of HSPCs with targeted gene insertion into the alpha globin locus. In preclinical studies, we observed a targeted insertion of a full HBB gene (which encodes the beta-globin protein) into the HBA1 locus at an approximately 40% rate in human beta-thalassemia patient-derived HSPCs, as shown in the figure at left below. Following the insertion of the HBB gene into the HBA1 locus, transplantation of these patient-derived cells into an immunodeficient mouse model resulted in long-term engraftment, as shown in the center figure below. Following differentiation of HSPCs into red blood cells, the beta globin-to-alpha globin expression ratio was approximately equivalent to the levels observed in patients with beta-thalassemia trait, as shown in the figure at right below. This targeted gene insertion approach, which explores inserting
27
the HBB gene into the HBA1 locus as a method to potentially treat beta-thalassemia, is different from the gene replacement approach used for our GPH102 program, which replaces the HBB gene via HDR to achieve a normal or beta-thalassemia trait genotype.

Figure: Targeted gene insertion of the HBA1 gene led to efficient gene insertion and expression in a mouse model
Given high rates of protein production from our gene-targeted cells, we believe that clinically relevant therapeutics can be developed with this approach that require only modest rates of engraftment and would not require standard myeloablative conditioning. We believe that this has the potential to expand the applicability of our targeted gene insertion technology to indications for which risks of random gene integration and chemotherapeutic myeloablative conditioning would be unacceptable.
We were previously advancing an early-stage research program leveraging this approach for the treatment of alpha-1 antitrypsin (AAT) deficiency, a severe inherited genetic disorder that affects approximately 60,000 people in the United States and can cause progressive lung and liver disease. AAT is caused by a genetic mutation in the SERAPINA1 gene, which causes the liver to produce insufficient amounts of AAT protein needed to protect the lungs so they can work normally. We believe this could offer a differentiated approach to treat AAT deficiency by using targeted gene insertion to permanently increase AAT protein production. This approach has the potential to permanently normalize circulating plasma levels of AAT with a one-time treatment, in combination with mild conditioning, and avoid potential issues of durability, immunogenicity or liver toxicity with liver-targeted approaches.
Other Future Opportunities for Targeted Gene Integration in Other Cell Types and Indications
Given these future opportunities leverage the same gene editing platform technology as nula-cel, we do not currently intend to pursue them.
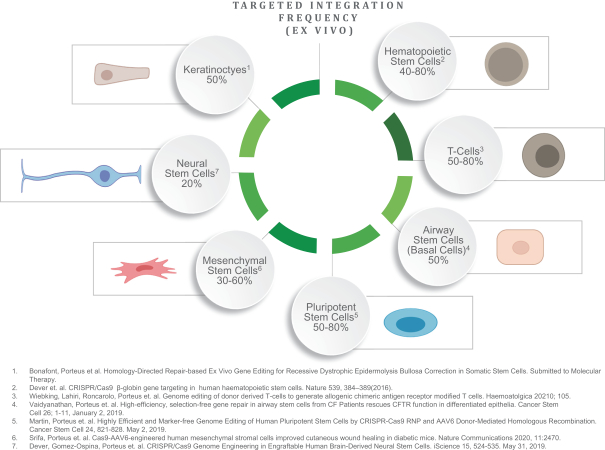
We had previously explored applications of our technology platform to develop potential therapies for a number of other genetic diseases including diseases involving the hematopoietic system and other lysosomal storage diseases. We believe that our targeted gene insertion technology, through its ability to lead to the controlled expression of any gene, also has potential to treat diseases outside of monogenic diseases such as the ability to integrate genes to produce next generation CAR effector therapies or myeloid cell therapies for autoimmune disease or oncology. Our high-efficiency gene editing technology has been shown using human cells and/or animal models to be applicable to a broad range of HSC-based indications (e.g., MPS I and Krabbe) as well as other tissues, such as airway stem cells (cystic fibrosis), neural stem cells, pluripotent stem cells and keratinocytes (wound healing).
28
Manufacturing
We currently have no commercial manufacturing capabilities. For our initial clinical programs, we planned to use qualified third-party contract manufacturing organizations with relevant manufacturing experience in genetic medicines. We have established manufacturing processes for nula-cel and have established relationships with third-party manufacturers with capabilities to manufacture the necessary Drug Substance and Drug Product in accordance with current Good Manufacturing Practices (cGMP). We plan to continue to rely on qualified third-party organizations to produce or process bulk compounds, formulated compounds, viral vectors or engineered cells for IND-supporting activities and early-stage clinical trials if applicable. We expect that commercial quantities of any compound, vector, or engineered cells that we may seek to develop will be manufactured in facilities and by processes that comply with cGMP and relevant health authority regulations. We had previously planned that at the appropriate time in the product development process, we would determine whether to establish our own manufacturing facilities or continue to rely on third parties to manufacture commercial quantities of any products that we may successfully develop. Outside of the United States and Europe, where appropriate, we may elect in the future to utilize strategic partners, distributors or contract sales forces to assist in the commercialization of our products, or, in certain instances, we may consider building our own commercial infrastructure.
Competition
The gene therapy and gene editing fields are characterized by rapidly advancing technologies, intense competition, and a strong emphasis on intellectual property and proprietary products. While we believe that our technology, development experience, and scientific knowledge provide us with competitive advantages, we currently face, and will continue to face, competition from many different sources, including major pharmaceutical, specialty pharmaceutical and biotechnology companies, as well as academic institutions, government agencies and private and public research institutions. For any products that we may ultimately commercialize, not only will we compete with any existing therapies and those therapies currently in development, but we will also have to compete with new therapies that may become available in the future. Key competitive factors affecting the commercial success of our gene therapies are likely to be efficacy, safety and tolerability profile, reliability, convenience, price and reimbursement.
29
We compete in the segments of the pharmaceutical, biotechnology, and other related markets that utilize technologies encompassing genomic medicines to create therapies, including gene editing and gene therapy. There are several other companies advancing gene editing and gene therapy product candidates in preclinical or clinical development in sickle cell disease, including Beam Therapeutics Inc., bluebird bio, Inc., Cellectis SA, CRISPR Therapeutics AG, and Editas Medicine, Inc. Companies advancing gene editing and gene therapy programs in beta-thalassemia include bluebird bio, Inc., CRISPR Therapeutics AG, and Edigene Inc. Companies advancing gene therapy programs in XSCID include Mustang Bio, Inc. Companies advancing gene therapy programs in Gaucher disease include AVROBio, Inc. and Freeline Therapeutics Holdings plc. Companies advancing gene editing and gene therapy programs in preclinical development for AAT deficiency include Beam Therapeutics Inc., Editas Medicine, Inc., Intellia Therapeutics, Inc., Krystal Biotech Inc., Apic Bio Inc., and LogicBio Therapeutics Inc. Companies combining CRISPR with HDR (homology directed repair) include CRISPR Therapeutics AG, which, for oncology applications, inserts a chimeric antigen receptor (CAR) construct into the TCR alpha constant (TRAC) locus in T-cells using HDR. Additionally, an academic collaboration between the University of California, San Francisco and the University of California, Los Angeles is seeking to correct the sickle cell mutation using CRISPR followed by delivery of a single-stranded oligonucleotide DNA donor to potentially harness HDR. Because these competitors, as well as other companies and research institutions, hold numerous patents in this field, it is possible that these or other third parties could allege they have patent rights encompassing our product candidates, technologies or methods. For more information regarding competition and intellectual property, please see the section titled “Risk Factors—Risks Related to Our Intellectual Property.”
Mergers and acquisitions in the pharmaceutical and biotechnology industries may result in even more resources being concentrated among a small number of our competitors. Accordingly, our competitors may be more successful than we may be in obtaining FDA approval for drugs and achieving widespread market acceptance. Our competitors’ products may be more effective, or more effectively marketed and sold, than any product we may commercialize and may render our gene therapies obsolete or non-competitive before we can recover the expenses of developing and commercializing any of our gene therapies. Our competitors may also obtain FDA or other regulatory approval for their products more rapidly than we may obtain approval for ours. We anticipate that we will face intense and increasing competition as new drugs enter the market and advanced technologies become available. Finally, the development of new treatment methods for the diseases we are targeting could render our gene therapies non-competitive or obsolete.
Intellectual Property
Our success depends in part on our ability to obtain and maintain proprietary protection for our platform technology, our programs, and know-how related to our business, defend and enforce our intellectual property rights, in particular, our patent rights, preserve the confidentiality of our trade secrets, and operate without infringing, misappropriating or otherwise violating any valid and enforceable intellectual property rights of others. We seek to protect our proprietary position by, among other things, exclusively licensing and filing patent applications related to our platform technology, existing and planned programs, and improvements that are important to the development of our business, where patent protection is available. Notwithstanding these efforts, we cannot be sure that patents will be granted with respect to any patent applications we have licensed or filed or may license or file in the future, and we cannot be sure that any patents we have licensed or patents that may be licensed or granted to us in the future will not be challenged, invalidated, or circumvented or that such patents will be commercially useful in protecting our technology. For more information regarding the risks related to our intellectual property, please see section titled “Risk Factors—Risks Related to Our Intellectual Property.”
Our wholly owned and our in-licensed patent applications cover various aspects of our genome editing platform and proprietary components, as well as our programs directed to genome modification using chemically modified guide RNAs, and non-genotoxic conditioning. We intend to continue to pursue, when possible, additional patent protection, including composition of matter, method of use, and process claims, directed to various aspects of our platform technology and the programs in our portfolio. We also have one option to license patent applications relating to stable genomic integration in primary cells with CRISPR-Cas and AAV, HSC insertion in alpha globin loci, and HDR correction of IL2RG for treatment of XSCID as well as a second option to license patent applications relating to gene integration in a safe harbor locus CCR5 to correct metabolic diseases, treatment and correction of immunodeficiency conditions, treatment of cystic fibrosis, and treatment of diseases relating via targeting and correcting the alpha globin locus. As of March 1, 2023, we owned two pending U.S. patent applications, two pending PCT patent applications, and five pending provisional patent applications. One patent family (comprising pending U.S. and PCT patent applications) relates to various aspects of treating beta-thalassemia, including specific gene cassettes and sequences for targeted insertion into particular locations in the HBB gene, gene editing components and compositions thereof for editing the HBB gene, and methods of using such compositions for modifying a patient’s cells and other gene therapies. If a U.S. patent should issue from this family, and if the appropriate maintenance fees are paid, the U.S. patent would be expected to expire in 2042, excluding any additional term for patent term adjustments or patent term extensions. A second patent family (comprising pending U.S. and PCT patent applications) relates to methods to genetically engineer hematopoietic stem and progenitor cells for the expression of therapeutic proteins, including related gene editing components and therapeutic compositions and methods. If a U.S. patent should issue from this family, and if the appropriate maintenance fees are paid, the U.S. patent would be expected to expire in 2042, excluding any additional term for patent term adjustments or patent term extensions. Two pending provisional patent applications relate to our Non-Genotoxic HSC Targeted Conditioning (NGTC) program, including compositions and methods for use in non-genotoxic HSC targeted antibody-based bone-marrow conditioning regimens. If a U.S. patent should issue claiming priority to either or both of these provisional applications, the U.S. patent(s) would be expected to expire in 2043, excluding
30
any additional term for patent term adjustments or patent term extensions. Two additional pending provisional patent applications relate to methods for genotyping gene-edited hematopoietic stem cells. If a U.S. patent should issue claiming priority to either or both of these provisional applications, the U.S. patent(s) would be expected to expire in 2043, excluding any additional term for patent term adjustments or patent term extensions. An additional pending provisional patent application relates to compositions and methods for enhancing homology directed repair (HDR)-mediated gene editing. If a U.S. patent should issue claiming priority to this provisional application, the U.S. patent(s) would be expected to expire in 2043, excluding any additional term for patent term adjustments or patent term extensions.
As of March 1, 2023, we in-licensed from Stanford two issued U.S. patents and two pending U.S. patent applications, issued patents in Australia, Europe and China, and pending patent applications in Australia, Canada, China, Japan and South Korea directed to methods of genome modification using chemically modified guide RNA in primary cells. The in-licensed patents and patent applications, which are jointly owned by Stanford and Agilent, also relate to methods of using such genome modifications for therapeutic indications such as SCD and thalassemia. Our current in-licensed patents and patent applications from Stanford, if the appropriate maintenance fees are paid, are expected to expire 2036, excluding any additional term for patent term adjustments or patent term extensions. The in-licensed European patent is currently subject to an opposition proceeding at the European Patent Office (EPO) Opposition Division, initiated by multiple opponents.
As of March 1, 2023, we in-licensed two U.S. patents, two U.S. patent applications, and patent applications in Australia, Canada, China, Europe, Japan and South Korea directed to compositions involving high-fidelity nucleases, gene editing systems using mutant Cas9 nucleases, and improved methods of gene editing thereof from IDT. Our current in-licensed patent and patent applications from IDT, if the appropriate maintenance fees are paid, are expected to expire in 2037, excluding any additional term for patent term adjustments or patent term extensions.
For more information regarding our licensed patent applications, please see the sections titled “Our Material Agreements” and “Risk Factors—Risks Related to Our Intellectual Property.”
The term of individual patents depends upon the legal term for patents in the countries in which they are granted. In most countries, including the United States, the patent term is 20 years from the earliest claimed filing date of a non-provisional patent application in the applicable country. However, the actual protection afforded by a patent varies from country to country, and depends upon many factors, including the type of patent, the scope of its coverage, the availability of regulatory-related extensions, the availability of legal remedies in a particular country and the validity and enforceability of the patent. In the United States, a patent’s term may, in certain cases, be lengthened by patent term adjustment (PTA) which compensates a patentee for administrative delays by the USPTO in examining and granting a patent, or may be shortened (e.g., if a patent is terminally disclaimed over a commonly owned patent having an earlier expiration date). In some instances, such a PTA may result in a U.S. patent term extending beyond 20 years from the earliest date of filing a non-provisional patent application related to the U.S. patent. Patent term extensions (PTE) under the Drug Price Competition and Patent Term Restoration Act of 1984, commonly known as the Hatch-Waxman Act, are also possible for patents that cover an FDA-approved drug as compensation for the patent term lost during the FDA regulatory review process. The Hatch-Waxman Act permits a PTE of up to five years beyond the expiration of the patent. The length of the PTE is related to the length of time the drug is under regulatory review. PTE cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval and only one patent applicable to an approved drug, a method for using it, or a method of manufacturing it, may be extended. Similar provisions are available in Europe and certain other jurisdictions to extend the term of a patent that covers an approved drug. In the future, if our products receive regulatory approval, we may be eligible to apply for PTEs on patents covering such products, however there is no guarantee that the applicable authorities, including the FDA in the United States, will agree with our assessment of whether such PTE should be granted, and if granted, the length of such PTE. For more information regarding the risks related to our intellectual property, please see section titled “Risk Factors—Risks Related to Our Intellectual Property.”
We also rely on trade secrets, know-how, continuing technological innovation, and confidential information to develop and maintain our proprietary position and protect aspects of our business that are not amenable to, or that we do not consider appropriate for, patent protection. We seek to protect our proprietary technology and processes, in part, by confidentiality agreements with our employees, consultants, scientific advisors, and contractors. We also seek to preserve the integrity and confidentiality of our data and trade secrets by maintaining physical security of our premises and physical and electronic security of our information technology systems. While we have implemented measures to protect and preserve our trade secrets, such measures can be breached, and we may not have adequate remedies for any such breach. In addition, our trade secrets may otherwise become known or be independently
31
discovered by competitors. For more information regarding the risks related to our intellectual property, please see section titled “Risk Factors—Risks Related to Our Intellectual Property.”
Our Material Agreements
Service Agreement with WuXi Advanced Therapies Inc.
In November 2022, we entered into a Master Development and Manufacturing Services Agreement (“WuXi Agreement”) with WuXi Advanced Therapies Inc. (“WuXi”), pursuant to which we agreed that, for a term of five years (which is extendable by mutual written agreement of the parties) and subject to certain conditions, WuXi and certain of its affiliates will provide product candidate development and manufacturing services on a project-by-project basis. Such services and their pricing terms will be agreed upon between WuXi and us pursuant to one or more work orders executed in accordance with the WuXi Agreement. The WuXi Agreement may be terminated by either party for any reason upon at least 90 days written notice, and we may terminate any work order under the WuXi Agreement upon at least 30 days written notice. In addition, each party has the ability to terminate the WuXi Agreement upon the occurrence of certain conditions.
The WuXi Agreement includes customary provisions relating to indemnification, intellectual property protection, confidentiality, remedies, and representations and warranties, as well as certain quality requirements.
On November 7, 2022, we entered into a work order under the WuXi Agreement under which we will receive services for nula-cel, manufacturing engineering test runs, the use of a manufacturing suite at WuXi’s GMP facility located in Philadelphia, PA, and Phase 1/2 CEDAR clinical development and manufacturing of nula-cel.
Exclusive License Agreement with the Board of Trustees of the Leland Stanford Junior University
In December 2020, we entered into an exclusive license agreement (the “License Agreement”), with The Board of Trustees of the Leland Stanford Junior University (Stanford), pursuant to which Stanford granted us a worldwide license to specified technology and patent rights to develop, manufacture and commercialize human prophylactic and therapeutic products. Other than with respect to specified, broadly applicable assays and procedures and subject to retained rights by Stanford, the license is exclusive with respect to human prophylactic and therapeutic products for the treatment of SCD, XSCID and beta thalassemia. The license is non-exclusive with respect to those broadly applicable assays and procedures and with respect to all human prophylactic and therapeutic products other than for the treatment of SCD, XSCID and beta thalassemia.
To date, pursuant to the License Agreement, we have paid an upfront license fee to Stanford of $50.0 thousand and issued to Stanford and its designees an aggregate of approximately 0.6 million shares of our common stock. The acquisition of the exclusive license, including patent rights and know-how, and clinical supplies was accounted for as an asset acquisition and as the acquired technology and inventories did not have an alternative use, the total consideration of $2.8 million was recorded as research and development expense in the statements of operations and comprehensive loss for the year ended December 31, 2020. We are obligated to pay Stanford an annual license maintenance fee on each anniversary of the effective date of the License Agreement. The annual license maintenance fee initially is $5.0 thousand and will increase to $50.0 thousand in three increments over the first seven anniversaries of the effective date of the License Agreement. After the first commercial sale of a product falling within the scope of the license (the “Licensed Product”), the annual license maintenance fee is $200.0 thousand.
In May 2021, we issued 640,861 shares of our common stock in connection with the License Agreement. Subsequently, in June 2021, related to the License Agreement, we repurchased 624,845 shares of our common stock from investors and founders.
We are required to share with Stanford a portion of any non-royalty income we receive from sublicensing the licensed patent rights or technology, subject to specified exclusions. With respect to sublicenses granted to products for the treatment of SCD, XSCID and beta thalassemia, the portion of sublicense income we must share with Stanford varies by indication and declines from between a mid-teen to a second quartile double-digit percentage prior to the filing of an IND to between a high single-digit to very low double-digit percentage upon achievement of a specified clinical milestone. With respect to sublicenses granted under the licensed technology rights and not licensed patent rights, the portion of sublicense income shared with Stanford declines from between a mid-single-digit and very low double-digit percentage prior to the filing of an IND to a low single-digit percentage after filing of an IND.
We are obligated to make payments to Stanford with respect to each Licensed Product of up to an aggregate of $12.8 million upon the achievement of certain development, regulatory and commercial milestones. Such amounts are payable only once upon the first occurrence of a particular milestone event with respect to each Licensed Product and only once with respect to each new indication covered by any of the Licensed Products.
We also are obligated to pay Stanford low single-digit royalties based on worldwide annual net sales of any Licensed Product, subject to specified reductions. We will be obligated to continue to pay royalties on a Licensed Product-by-Licensed Product and country-by-country basis, until the latest of (i) the expiration of the last valid claim under the licensed patents that covers the sale or manufacture of such Licensed Product in such country, (ii) the expiration of any period of regulatory exclusivity with respect to such
32
Licensed Product in such country or (iii) the expiration of ten years after the first commercial sale of such Licensed Product in such country.
The term of the License Agreement expires on the later of (i) the expiration of the last patent or abandonment of the last patent application within the license patent rights or (ii) the expiration of all royalty terms with respect to Licensed Products. The License Agreement may be terminated by us at will or by Stanford if we remain in breach of the License Agreement following a cure period to remedy the breach.
We are required to use diligent efforts to manufacture, market and sell Licensed Products for the treatment of each of SCD, XSCID and beta thalassemia. In addition, we are required to achieve specified milestones by specified dates with respect to Licensed Products for the treatment of each of SCD, XSCID and beta thalassemia. If we fail to satisfy our diligence obligations, Stanford may terminate the License Agreement for our breach. For more details on the License Agreement, please see Note 6 of the Notes to the Financial Statements.
Option Agreements with Stanford
First Option Agreement
In January 2021, we entered into an option agreement (the “First Option Agreement”) with Stanford, pursuant to which Stanford granted us the right to obtain a license to specified patent rights relating to human prophylactic and therapeutic products. The option may be extended to specified technology at a later date and upon our agreement with Stanford. We may exercise the option in whole or in part to obtain a license under one or more of the optioned patent rights. Subject to retained rights by Stanford, the license is exclusive with respect to human prophylactic and therapeutic products for the treatment of SCD, XSCID and beta thalassemia and non-exclusive with respect to all other human prophylactic and therapeutic products.
Pursuant to the First Option Agreement, we agreed to grant Stanford 132,137 shares of our common stock if we exercise the option and execute and deliver an amendment to the License Agreement that incorporates the optioned patent rights and any optioned technology. Other than such shares of our common stock and a license execution fee of $10.0 thousand if we exercise the option with respect to a particular optioned patent right, no additional payments have been or will be made by us to Stanford under the First Option Agreement or upon the execution of an amendment to the License Agreement that incorporates the optioned patent rights and any optioned technology. The terms of the License Agreement will apply to any Licensed Products falling within the patent rights and technology licensed by us upon exercise of the option.
The term of the First Option Agreement expires 18 months after its effective date, subject to our right to extend such expiration date by up to an additional one-year periods upon notice to Stanford and by another additional one year upon the reasonable agreement of Stanford. The First Option Agreement will terminate if the License Agreement terminates. The First Option Agreement also may be terminated by us at will. On June 23, 2022, we exercised our right to extend the term of the First Option Agreement for an additional year. As of March 1, 2023, we had not exercised the option under the First Option Agreement and no fees have been paid for the First Option Agreement.
Second Option Agreement
In April 2021, we entered into another option agreement (the “Second Option Agreement”) with Stanford, pursuant to which Stanford granted us the exclusive right to obtain a license to specified patent rights relating to human prophylactic and therapeutic products. The option may be extended to specified technology at a later date and upon our agreement with Stanford. We may exercise the option in whole or in part to obtain a license under one or more of the optioned patent rights, subject to a specified waiting period with respect to certain specified patent rights. Subject to retained rights by Stanford and in the case of specified patent rights, the Department of Veterans Affairs, the license will be exclusive with respect to human prophylactic and therapeutic products for the treatment of Gaucher Disease, other diseases treated through insertion of a construct into the CCR5 locus, and diseases treated through insertion of a construct into the alpha globin locus. The license is non-exclusive with respect to all other human prophylactic and therapeutic products.
Pursuant to the Second Option Agreement, we agreed to pay Stanford option fees in an aggregate amount of $30.0 thousand over the term of the option. If we exercise the option with respect to a particular optioned patent right, Stanford and we would negotiate in good faith the terms of a license agreement or an amendment to the License Agreement that incorporates the optioned patent rights and any optioned technology. The terms of the license agreement or amendment could include additional payments to Stanford in excess of those set forth in the License Agreement.
The term of the Second Option Agreement expires 12 months after its effective date, subject to our right to extend such expiration date by two additional one-year periods upon notice to and the reasonable agreement of Stanford. The Second Option Agreement may be terminated by us at will or by Stanford if we remain in breach of the Second Option Agreement following a cure period to remedy the breach. The Second Option Agreement also will terminate automatically in the event of a filing of a bankruptcy petition by or against us. On April 13, 2022, we entered into an amendment to the Second Option Agreement which extended the term for an additional year
33
and the maintenance fee of $10.0 thousand was paid in the three and six months ended June 30, 2022. As of March 1, 2023, we had not exercised the option under the Second Option Agreement and no fees have been paid for the Second Option Agreement.
We are required to use diligent efforts to conduct research on potential commercial applications of the optioned patents and any optioned technology. In addition, we are required to use reasonable efforts to achieve specified milestones during the term of the Second Option Agreement with respect to products incorporating two of therapeutic approaches covered by the optioned patent rights. Our diligence obligations are subject to good faith discussions regarding their modification upon any extension of the term of the Second Option Agreement by us. If we fail to satisfy our diligence obligations Stanford may terminate the Second Option Agreement for our breach.
License Agreement with Integrated DNA Technologies, Inc.
In June 2021, we entered into a License Agreement (the “IDT License Agreement”) with Integrated DNA Technologies, Inc. (IDT). Pursuant to the IDT License Agreement, IDT granted to us and our affiliates a worldwide, non-exclusive, sublicensable license to research and develop products incorporating HiFi Cas9 protein variants for use in human therapeutic applications for SCD, XSCID and Gaucher disease (the Field) and a worldwide, exclusive, sublicensable license to commercialize such products in the Field. We were also granted the right to expand the licensed Field to include human therapeutic applications in the additional fields of beta thalassemia disorder and lysosomal storage disorders upon the payment of an exercise fee in the amount of $0.5 million per additional field or $1.0 million for both additional fields.
We are solely responsible for the development, manufacture, regulatory approval and commercialization of the products in the Field and are required to use commercially reasonable efforts to achieve certain regulatory and commercial milestones with respect to licensed products.
In the event we do not achieve the applicable milestones within a specified time period, then, except with respect to the field of human therapeutic applications for SCD for which we had previously filed an IND, the exclusive license granted to us described above will immediately convert to a non-exclusive, non-sublicensable license, and all sublicenses granted by us to any sublicensees will immediately terminate.
In consideration of the licenses and rights granted to us under the IDT License Agreement, we agreed to pay to IDT an upfront payment in the amount of $3.0 million and up to $5.3 million (or $8.8 million if we expand the Field as described above to include both the beta thalassemia and lysosomal storage disorders fields) in total regulatory milestone payments. Each regulatory milestone payment is payable once on an indication-by-indication basis. In addition, we have agreed to pay IDT a low single-digit royalty on the net sales of products, subject to reductions in specified circumstances, and a low double digit percentage payment for certain sublicense revenue, which is also subject to reduction in the event a sublicense includes other patent rights that are not patents licensed from IDT. As of December 31, 2022 and 2021, we have not achieved any of the regulatory milestones and have only paid the upfront license payment of $3.0 million.
The IDT License Agreement remains in effect on a country-by-country and product-by-product basis until the expiration of the royalty term for such product in such jurisdiction. We and IDT each have the right to terminate the IDT License Agreement for the other party’s material breach of its obligations under the IDT License Agreement, subject to specified rights to cure. Additionally, we may terminate the IDT License Agreement for any reason.
Our current in-licensed patent and patent applications from IDT, if the appropriate maintenance fees are paid, are expected to expire in 2037, excluding any additional term for patent term adjustments or patent term extensions.
The IDT License Agreement includes customary representations and warranties by each party as are customarily found in transactions of this nature, including as to the licensed intellectual property. The IDT License Agreement also provides for certain mutual indemnities for breaches of representations, warranties and covenants.
Government Regulation
The FDA and other regulatory authorities at federal, state and local levels, as well as in foreign countries, extensively regulate, among other things, the research, development, testing, manufacture, quality control, import, export, safety, effectiveness, labeling, packaging, storage, distribution, record keeping, approval, advertising, promotion, marketing, post-approval monitoring and post-approval reporting of biologics such as those we have been developing.
U.S. Biologics Regulation
In the United States, the FDA regulates biological products under the Federal Food, Drug, and Cosmetic Act (FDCA), and the Public Health Service Act (PHSA), and their implementing regulations. Biological products are also subject to other federal, state, local and foreign statutes and regulations. We, along with third-party contractors, will be required to navigate the various preclinical, clinical and commercial approval requirements of the governing regulatory agencies of the countries in which we wish to conduct studies or seek approval or licensure of our product candidates. Failure to comply with the applicable U.S. requirements at any time during the product development process, approval process or after approval, may result in delays to the conduct of a study, regulatory review and
34
approval or subject an applicant to administrative or judicial sanctions. These sanctions could include, among other actions, the FDA’s refusal to approve pending applications, withdrawal of an approval, license suspension or revocation, refusal to allow an applicant to proceed with clinical trials, imposition of a clinical hold, issuance of untitled or warning letters, product recalls or withdrawals from the market, product seizures, total or partial suspension of production or distribution, injunctions, fines, refusals of government contracts, restitution, disgorgement of profits, or civil or criminal investigations or penalties.
Our product candidates must be approved by the FDA through the Biologics License Application (BLA), process before they may be legally marketed in the United States. The process required by the FDA before biological product candidates may be marketed in the United States generally involves the following:
Preclinical and Clinical Development
Prior to beginning the first clinical trial with a product candidate, a sponsor must submit an IND to the FDA. An IND is a request for authorization from the FDA to administer an investigational new drug product to humans. The central focus of an IND submission is on the general investigational plan and the protocol or protocols for preclinical studies and clinical trials. The IND also includes results of animal and in vitro studies assessing the toxicology, pharmacokinetics, pharmacology and pharmacodynamic characteristics of the product, chemistry, manufacturing and controls information, and any available human data or literature to support the use of the investigational product. An IND must become effective before human clinical trials may begin. The IND automatically becomes effective 30 days after receipt by the FDA, unless the FDA, within the 30-day period, raises safety concerns or questions about the proposed clinical trial. In such a case, the IND may be placed on clinical hold and the IND sponsor and the FDA must resolve any outstanding concerns or questions before the clinical trial can begin. The FDA may also impose clinical holds on a product candidate at any time before or during clinical trials due to safety concerns, non-compliance, or other issues affecting the integrity of the trial. Submission of an IND therefore may or may not result in FDA authorization to begin a clinical trial and, once begun, issues may arise that could cause the trial to be suspended or terminated.
In addition to the submission of an IND to the FDA before initiation of a clinical trial in the United States, certain human clinical trials involving recombinant or synthetic nucleic acid molecules are subject to oversight at the local level as set forth in the National Institutes of Health (NIH), Guidelines for Research Involving Recombinant or Synthetic Nucleic Acid Molecules (NIH Guidelines). Specifically, under the NIH Guidelines, supervision of human gene transfer trials includes evaluation and assessment by an Institutional Biosafety Committee (IBC), a local institutional committee that reviews and oversees research utilizing recombinant or synthetic nucleic acid molecules at that institution. The IBC assesses the safety of the research and identifies any potential risk to public health or the environment, and such review may result in some delay before initiation of a clinical trial. While the NIH Guidelines are not mandatory unless the research in question is being conducted at or sponsored by institutions receiving NIH funding for recombinant or synthetic nucleic acid molecule research, many companies and other institutions not otherwise subject to the NIH Guidelines voluntarily follow them.
Clinical trials involve the administration of the investigational product to human subjects under the supervision of qualified investigators, generally physicians not employed by or under the trial sponsor’s control, in accordance with GCPs, which include the
35
requirement that all research subjects provide their informed consent for their participation in any clinical trial. Clinical trials are conducted under protocols detailing, among other things, the objectives of the study, dosing procedures, subject selection and exclusion criteria, and the parameters to be used in monitoring safety and the effectiveness criteria to be evaluated. A separate submission to the existing IND must be made for each successive clinical trial conducted during product development and for any subsequent protocol amendments. Furthermore, an independent IRB or ethics committing at or servicing each site at which the clinical trial will be conducted must review and approve the plan for any clinical trial before the clinical trial begins at that site, and must monitor the study until completed. An IRB is charged with protecting the welfare and rights of trial participants and considers such items as whether the risks to individuals participating in the clinical trials are minimized and are reasonable in relation to anticipated benefits. The IRB also approves the informed consent form that must be provided to each clinical trial subject or his or her legal representative. Regulatory authorities, the IRB or the sponsor may suspend a clinical trial at any time on various grounds, including a finding that the subjects are being exposed to an unacceptable health risk or that the trial is unlikely to meet its stated objectives. Some studies also include oversight by an independent group of qualified experts organized by the clinical study sponsor, known as a data safety monitoring board, which provides authorization for whether or not a study may move forward at designated check points based on access to certain data from the study and may halt the clinical trial if it determines that there is an unacceptable safety risk for subjects or other grounds, such as no demonstration of efficacy.
There are also requirements governing the reporting of ongoing preclinical studies and clinical trials and clinical study results to public registries. Sponsors of certain clinical trials of FDA-regulated products, including biologics, are required to register and disclose certain clinical trial information, which is publicly available at www.clinicaltrials.gov.
Clinical trials are typically conducted in three sequential phases that may overlap or be combined:
When these phases overlap or are combined, the trials may be referred to as Phase 1/2 or Phase 2/3.
In some cases, the FDA may require, or companies may voluntarily pursue, additional clinical trials after a product is approved to gain more information about the product. These so-called Phase 4 studies may be made a condition to approval of the BLA. These trials are used to gain additional experience from the treatment of patients in the intended therapeutic indication and further document clinical benefit in the case of drugs approved under accelerated approval regulations. Failure to exhibit due diligence with regard to conducting Phase 4 clinical trials could result in withdrawal of approval for products.
Concurrent with clinical trials, companies may complete additional animal studies and develop additional information about the characteristics of the product candidate, and must finalize a process for manufacturing the product in commercial quantities in accordance with cGMP requirements. The manufacturing process must be capable of consistently producing quality batches of the product candidate and, among other things, must develop methods for testing the identity, strength, quality and purity of the final product, or for biologics, the safety, purity and potency. Additionally, appropriate packaging must be selected and tested and stability studies must be conducted to demonstrate that the product candidate does not undergo unacceptable deterioration over its shelf life.
During all phases of clinical development, regulatory agencies require extensive monitoring and auditing of all clinical activities, clinical data, and clinical study investigators. Progress reports detailing the results of the clinical trials, among other information, must be submitted at least annually to the FDA, and written IND safety reports must be submitted to the FDA and the investigators for serious and unexpected suspected adverse events, findings from other studies suggesting a significant risk to humans exposed to the biologic, findings from animal or in vitro testing that suggest a significant risk for human subjects, and any clinically important increase in the rate of a serious suspected adverse reaction over that listed in the protocol or investigator brochure.
The FDA, the sponsor or the IRB or may suspend a clinical study at any time on various grounds, including a finding that the research patients or patients are being exposed to an unacceptable health risk. Similarly, an IRB can suspend or terminate approval of a clinical study at its institution if the clinical study is not being conducted in accordance with the IRB’s requirements or if the biological product candidate has been associated with unexpected serious harm to patients. Additionally, if the trial is being overseen by a data safety monitoring board or committee, this group may recommend halting the clinical trial if it determines that there is an unacceptable safety risk for subjects or on other grounds, such as interim data suggesting a lack of efficacy.
36
BLA Submission and Review
Assuming successful completion of all required testing in accordance with all applicable regulatory requirements, the results of product development, preclinical studies and clinical trials are submitted to the FDA as part of a BLA requesting approval to market the product candidate for one or more indications. The BLA must include all relevant data available from preclinical studies and clinical trials, including negative or ambiguous results as well as positive findings, together with detailed information relating to the product candidate’s chemistry, manufacturing, controls, and proposed labeling, among other things. Under the Prescription Drug User Fee Act (PDUFA), as amended, each BLA must be accompanied by a significant application user fee to the FDA, unless a waiver or exemption applies, which is adjusted on an annual basis. The FDA has sixty days from the applicant’s submission of a BLA to either issue a refusal to file letter or accept the BLA for filing, indicating that it is sufficiently complete to permit substantive review. The FDA has substantial discretion in the approval process and may refuse to accept any application or decide that the data is insufficient for approval, and may require additional preclinical, clinical or other studies before it accepts the filing.
Once a BLA has been accepted for filing, the FDA’s goal is to review standard applications within ten months after it accepts the application for filing, or, if the application qualifies for priority review, six months after the FDA accepts the application for filing. In both standard and priority reviews, the review process may be significantly extended by FDA requests for additional information or clarification. The FDA reviews a BLA to determine, among other things, whether a product candidate is safe, pure and potent for its intended use, and whether the facility in which it is manufactured, processed, packed or held meets standards designed to assure and preserve the product’s identity, safety, strength, quality, and purity. The FDA may convene an advisory committee, typically a panel that includes clinicians and other experts, to provide clinical insight on applications which present difficult questions of safety or efficacy and to review, evaluation and a recommendation as to whether the application should be approved and under what conditions. The FDA is not bound by the recommendations of an advisory committee, but it considers such recommendations carefully when making decisions.
Before approving a BLA, the FDA will conduct a pre-approval inspection of the facility or facilities where the product is manufactured to determine whether the facilities comply with cGMPs. The FDA will not approve an application unless it determines that the manufacturing processes and facilities are in compliance with cGMP requirements and adequate to assure consistent production of the product within required specifications. Additionally, before approving a BLA, the FDA will typically audit data from clinical trials to ensure compliance with GCPs. If the FDA determines that the application, manufacturing process or manufacturing facilities are not acceptable, it will outline the deficiencies in the submission and often will request additional testing or information. Notwithstanding the submission of any requested additional information, the FDA ultimately may decide that the application does not satisfy the regulatory criteria for approval.
After the FDA evaluates a BLA and conducts inspections of manufacturing facilities where the investigational product and/or its drug substance will be manufactured, the FDA may issue an approval letter or a Complete Response letter. An approval letter authorizes commercial marketing of the product with specific prescribing information for specific indications. A Complete Response letter indicates that the review cycle of the application is complete and the application will not be approved in its present form. A Complete Response Letter usually describes all of the deficiencies that the FDA has identified in the BLA, except that where the FDA determines that the data supporting the application are inadequate to support approval, the FDA may issue the Complete Response letter without first conducting required inspections, testing submitted product lots and/or reviewing proposed labeling. In issuing the Complete Response letter, the FDA may recommend actions that the applicant might take to place the BLA in condition for approval, including requests for additional information or clarification, which may include the potential requirement for additional clinical studies and/or other significant and time-consuming requirements related to preclinical studies and manufacturing. If a Complete Response Letter is issued, the applicant may either resubmit the BLA, addressing all of the deficiencies identified in the letter, withdraw the application or request a hearing. Even if such data and information is submitted, the FDA may delay or refuse approval of a BLA if applicable regulatory criteria are not satisfied, require additional testing or information and/or require post-marketing testing and surveillance to monitor safety or efficacy of a product.
If regulatory approval of a product is granted, such approval will be granted for a particular indication(s) and may entail limitations on the indicated uses for which such product may be marketed. Further, the FDA may require that certain contraindications, warnings or precautions be included in the product labeling or may condition the approval of the BLA on other changes to the proposed labeling, development of adequate controls and specifications, or a commitment to conduct post-market testing or clinical trials and surveillance to monitor the effects of approved products. The FDA may also place other conditions on approvals including the requirement of a Risk Evaluation and Mitigation Strategy (REMS), to assure the benefits of the product outweigh its risks. A REMS is a safety strategy to manage a known or potential serious risk associated with a product and to enable patients to have continued access to such medicines by managing their safe use, and could include medication guides, physician communication plans, or elements to assure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. The FDA also may condition approval on, among other things, changes to proposed labeling or the development of adequate controls and specifications. Any of these limitations on approval or marketing could restrict the commercial promotion, distribution, prescription or dispensing of products. Once approved, the FDA may withdraw the product approval if compliance with pre- and post-marketing requirements is not maintained or if problems occur after the product reaches the marketplace. The FDA may require one or more Phase 4 post-market studies and surveillance to
37
further assess and monitor the product’s safety and effectiveness after commercialization, and may limit further marketing of the product based on the results of these post-marketing studies.
Expedited Development and Review Programs
The FDA offers a number of expedited development and review programs for qualifying product candidates. The fast track program is intended to expedite or facilitate the process for reviewing new product candidates that meet certain criteria. Specifically, product candidates are eligible for fast track designation if they are intended to treat a serious or life-threatening disease or condition and nonclinical or clinical data demonstrate the potential to address unmet medical needs for the disease or condition. Fast track designation applies to the combination of the product and the specific indication for which it is being studied. A sponsor may request fast track designation of a product candidate concurrently with, or at any time after, submission of an IND, and the FDA must determine if the product candidate qualifies for such designation within 60 day of receipt of the sponsor’s request. The sponsor of a fast track product has opportunities for frequent interactions with the FDA review team during product development and, once a BLA is submitted, the product may be eligible for priority review. A fast track product may also be eligible for rolling review, where the FDA may consider for review sections of the BLA on a rolling basis before the complete application is submitted, if the sponsor provides a schedule for the submission of the sections of the BLA, the FDA agrees to accept sections of the BLA and determines that the schedule is acceptable, and the sponsor pays any required user fees upon submission of the first section of the BLA.
A product candidate intended to treat a serious or life-threatening disease or condition may also be eligible for breakthrough therapy designation to expedite its development and review. A product candidate can receive breakthrough therapy designation if it is intended to treat a serious or life-threatening disease or condition and preliminary clinical evidence indicates that the product, alone or in combination with one or more other drugs or biologics, may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints, such as substantial treatment effects observed early in clinical development. A sponsor may request that a product candidate be designated as a breakthrough therapy concurrently with, or at any time after, the submission of an IND, and the FDA must determine if the product candidate qualifies for breakthrough therapy designation within 60 days of receipt of the sponsor’s request. The benefits of breakthrough therapy designation includes all of the fast track program features, as well as more intensive FDA interaction and guidance beginning as early as Phase 1 and an organizational commitment to expedite the development and review of the product, including involvement of senior managers and experienced review staff in a cross-disciplinary review.
As part of the 21st Century Cures Act, Congress amended the FDCA to create an expedited development program for RMATs, which include cell therapies, therapeutic tissue engineering products, human cell and tissue products, and combination products using any such therapies or products. Gene therapies, including genetically modified cells that lead to a sustained effect on cells or tissues may meet the definition of a RMAT. The RMAT program is intended to facilitate efficient development and expedite review of RMATs, which are intended to treat, modify, reverse, or cure a serious or life-threatening disease or condition. A sponsor may request that FDA designate a product candidate as a regenerative medicine advanced therapy concurrently with or at any time after submission of an IND. FDA has 60 calendar days to determine whether the drug meets the criteria, including whether there is preliminary clinical evidence indicating that the drug has the potential to address unmet medical needs for a serious or life-threatening disease or condition. A new drug application or a BLA for a RMAT may be eligible for priority review or accelerated approval through (1) surrogate or intermediate endpoints reasonably likely to predict long-term clinical benefit or (2) reliance upon data obtained from a meaningful number of sites. Benefits of such designation include early interactions with FDA to discuss any potential surrogate or intermediate endpoint to be used to support accelerated approval. A RMAT that is granted accelerated approval and is subject to post-approval requirements may fulfill such requirements through the submission of clinical evidence, clinical studies, patient registries, or other sources of real-world evidence, such as electronic health records; the collection of larger confirmatory data sets; or post-approval monitoring of all patients treated with such therapy prior to its approval.
Any marketing application for a product candidate submitted to the FDA for approval, including a product candidate with a fast track designation and/or breakthrough therapy designation, may be eligible for other types of FDA programs intended to expedite the FDA review and approval process, such as priority review and accelerated approval. A product candidate is eligible for priority review if it has the potential to provide a significant improvement in the safety or effectiveness of the treatment, diagnosis or prevention of a serious disease or condition compared to available therapies. For original BLAs, priority review designation means the FDA’s goal is to take action on the marketing application within six months of the 60-day filing date. The FDA will attempt to direct additional resources to the evaluation of an application for a new drug or biological product designated for priority review in an effort to facilitate the review.
Additionally, product candidates studied for their safety and effectiveness in treating serious or life-threatening diseases or conditions may receive accelerated approval upon a determination that the product candidate generally provides a meaningful advantage over available therapies and demonstrates an effect on a surrogate endpoint that is reasonably likely to predict clinical benefit, or on a clinical endpoint that can be measured earlier than irreversible morbidity or mortality that is reasonably likely to predict an effect on irreversible morbidity or mortality or other clinical benefit, taking into account the severity, rarity, or prevalence of the condition and the availability or lack of alternative treatments. As a condition of accelerated approval, the FDA will generally require the sponsor to perform adequate and well-controlled post-marketing clinical trials to verify and describe the anticipated effect on irreversible morbidity or mortality or other clinical benefit. Under the Food and Drug Omnibus Reform Act of 2022 (FDORA), the FDA is now permitted to
38
require, as appropriate, that such trials be underway prior to approval or within a specific time period after the date of approval for a product granted accelerated approval. Sponsors are also required to send updates to the FDA every 180 days on the status of such studies, including progress toward enrollment targets, and the FDA must promptly post this information publicly. Under FDORA, the FDA has increased authority for expedited procedures to withdraw approval of a drug or indication approved under accelerated approval if, for example, the sponsor fails to conduct such studies in a timely manner and send the necessary updates to the FDA, or if a confirmatory trial fails to verify the predicted clinical benefit of the product. In addition, for products being considered for accelerated approval, the FDA generally requires, unless otherwise informed by the agency, that all advertising and promotional materials intended for dissemination or publication within 120 days of marketing approval be submitted to the agency for review during the pre-approval review period, which could adversely impact the timing of the commercial launch of the product.
Fast track designation, breakthrough therapy designation, RMAT designation, priority review and accelerated approval do not change the standards for approval but may expedite the development or approval process. Even if a product qualifies for one or more of these programs, the FDA may later decide that the product no longer meets the conditions for qualification or decide that the time period for FDA review or approval will not be shortened.
Orphan Drug Designation and Exclusivity
Under the Orphan Drug Act, the FDA may grant orphan designation to a biological product candidate intended to treat a rare disease or condition, which is a disease or condition that affects fewer than 200,000 individuals in the United States, or 200,000 or more than individuals in the United States for which there is no reasonable expectation that the cost of developing and making available in the United States a product for this type of disease or condition will be recovered from sales in the United States for that product. Orphan drug designation must be requested before submitting a BLA. After the FDA grants orphan drug designation, the generic identity of the therapeutic agent and its potential orphan use are disclosed publicly by the FDA. Orphan drug designation does not convey any advantage in, or shorten the duration of, the regulatory review or approval process.
If a product candidate that has orphan drug designation subsequently receives the first FDA approval for the disease or condition for which it has such designation, the product is entitled to orphan exclusivity, which means that the FDA may not approve any other applications, including a full BLA, to market the same product for the same indication for seven years from the date of such approval, except in limited circumstances, such as a showing of clinical superiority to the product with orphan exclusivity or if the holder of the orphan exclusivity cannot assure the availability of sufficient quantities of the orphan drug to meet the needs of patients with the disease or condition for which the drug was designated. Orphan exclusivity does not prevent the FDA from approving a different product for the same disease or condition, or the same product for a different disease or condition. Among the other benefits of orphan drug designation are tax credits for certain research and a waiver of the BLA application fee.
A designated orphan drug may not receive orphan exclusivity if it is approved for a use that is broader than the indication for which it received orphan designation. In addition, exclusive marketing rights in the United States may be lost if the FDA later determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantities of the product to meet the needs of patients with the rare disease or condition.
Pediatric Trials and Exclusivity
Under the Pediatric Research Equity Act (PREA), a BLA or supplement to a BLA must contain data to assess the safety and efficacy of the product for the claimed indications in all relevant pediatric subpopulations and to support dosing and administration for each pediatric subpopulation for which the product is safe and effective. The FDCA requires that a sponsor who is planning to submit a marketing application for a product that includes a new active ingredient, new indication, new dosage form, new dosing regimen or new route of administration submit an initial Pediatric Study Plan (PSP), within sixty days of an end-of-Phase 2 meeting or as may be agreed between the sponsor and FDA. The initial PSP must include an outline of the pediatric study or studies that the sponsor plans to conduct, including study objectives and design, age groups, relevant endpoints and statistical approach, or a justification for not including such detailed information, and any request for a deferral of pediatric assessments or a full or partial waiver of the requirement to provide data from pediatric studies along with supporting information. The FDA and the sponsor must reach agreement on the PSP. A sponsor can submit amendments to an agreed-upon initial PSP at any time if changes to the pediatric plan need to be considered based on data collected from nonclinical studies, early phase clinical trials, and/or other clinical development programs. The FDA may, on its own initiative or at the request of the applicant, grant deferrals for submission of data or full or partial waivers. Sponsors who conduct studies of their product candidate in children are eligible for pediatric exclusivity. Pediatric exclusivity, if granted, adds six months to existing exclusivity periods and patent terms. This six-month exclusivity, which attaches to the twelve-year exclusivity period for reference biologics, may be granted based on the voluntary completion of a pediatric trial in accordance with an FDA-issued “Written Request” for such a trial.
Rare Pediatric Disease Designation and Priority Review Vouchers
Under the FDCA, as amended, the FDA incentivizes the development of drugs and biologics that meet the definition of a “rare pediatric disease,” defined to mean a serious or life-threatening disease in which the serious of life-threatening manifestations primarily affect individuals aged from birth to 18 years and the disease affects fewer than 200,000 individuals in the United States or affects
39
200,000 or more in the United States and for which there is no reasonable expectation that the cost of developing and making in the United States a drug for such disease or condition will be received from sales in the United States of such drug. The sponsor of a product candidate for a rare pediatric disease may be eligible for a voucher that can be used to obtain a priority review for a subsequent human drug or biologic application after the date of approval of the rare pediatric disease drug product, referred to as a priority review voucher (PRV). A sponsor may request rare pediatric disease designation from the FDA prior to the submission of its NDA or BLA. A rare pediatric disease designation does not guarantee that a sponsor will receive a PRV upon approval of its NDA or BLA. Moreover, a sponsor who chooses not to submit a rare pediatric disease designation request may nonetheless receive a PRV upon approval of their marketing application if they request such a voucher in their original marketing application and meet all of the eligibility criteria. If a PRV is received, it may be sold or transferred an unlimited number of times. Congress has extended the PRV program until September 30, 2024, with the potential for PRVs to be granted until September 30, 2026.
Post-Approval Requirements
Any products manufactured or distributed pursuant to FDA approvals are subject to pervasive and continuing regulation by the FDA, including, among other things, requirements relating to record-keeping, reporting of adverse experiences, periodic reporting, product sampling and distribution, and advertising and promotion of the product. After approval, most changes to the approved product, such as adding new indications or other labeling claims or changes of the site of manufacture, are subject to prior FDA review and approval. There also are continuing user fee requirements, under which the FDA assesses an annual program fee for each product identified in an approved BLA.
The FDA regulations require that products be manufactured in specific approved facilities and in accordance with cGMPs. Biological product manufacturers and their subcontractors are required to register their establishments with the FDA and certain state agencies, and are subject to periodic unannounced inspections by the FDA and certain state agencies for compliance with cGMPs, which impose certain organizational, procedural and documentation requirements with respect to manufacturing and quality assurance activities. Changes to the manufacturing process are strictly regulated, and, depending on the significance of the change, may require prior FDA approval before being implemented. FDA regulations also require investigation and correction of any deviations from cGMPs and impose reporting requirements upon us and any third-party manufacturers that we may decide to use. BLA holders using contract manufacturers, laboratories or packagers are responsible for the selection and monitoring of qualified firms, and, in certain circumstances, qualified suppliers to these firms. These firms and, where applicable, their suppliers are subject to inspections by the FDA at any time, and the discovery of violative conditions, including failure to conform to cGMP, could result in enforcement actions that interrupt the operation of any such facilities or the ability to distribute products manufactured, processed or tested by them. Manufacturers and other parties involved in the drug supply chain for prescription drug products must also comply with product tracking and tracing requirements and for notifying the FDA of counterfeit, diverted, stolen and intentionally adulterated products or products that are otherwise unfit for distribution in the United States. Accordingly, manufacturers must continue to expend time, money and effort in the area of production and quality control to maintain compliance with cGMPs and other aspects of regulatory compliance.
The FDA may withdraw approval if compliance with regulatory requirements and standards is not maintained or if problems occur after the product reaches the market. Later discovery of previously unknown problems with a product, including adverse events of unanticipated severity or frequency, or with manufacturing processes, or failure to comply with regulatory requirements, may result in revisions to the approved labeling to add new safety information; imposition of post-market studies or clinical studies to assess new safety risks; or imposition of distribution restrictions or other restrictions under a REMS program. Other potential consequences include, among other things:
The FDA closely regulates the marketing, labeling, advertising and promotion of biological products. A company can make only those claims relating to safety and efficacy, purity and potency that are approved by the FDA and in accordance with the provisions of the approved label. However, companies may share truthful and not misleading information that is otherwise consistent with a product’s
40
FDA approved labeling. The FDA and other agencies actively enforce the laws and regulations prohibiting the promotion of off-label uses. Failure to comply with these requirements can result in, among other things, adverse publicity, warning letters, corrective advertising and potential civil and criminal penalties. Physicians may prescribe legally available products for uses that are not described in the product’s labeling and that differ from those tested by us and approved by the FDA. Such off-label uses are common across medical specialties. Physicians may believe that such off-label uses are the best treatment for many patients in varied circumstances. The FDA does not regulate the behavior of physicians in their choice of treatments. The FDA does, however, restrict a manufacturer’s communications on the subject of off-label use of their products.
U.S. Patent Term Restoration
Depending upon the timing, duration and specifics of the FDA approval of the use of our biological product candidates, some of our U.S. patents may be eligible for limited patent term extension under the Hatch-Waxman Amendments. The Hatch-Waxman Amendments permit a patent restoration term of up to five years as compensation for patent term lost during product development and the FDA regulatory review process. However, patent term restoration cannot extend the remaining term of a patent beyond a total of 14 years from the product’s approval date. The patent term restoration period is generally one-half the time between the effective date of an IND and the submission date of a BLA plus the time between the submission date of a BLA and the approval of that application. Only one patent applicable to an approved biological product is eligible for the extension and the application for the extension must be submitted prior to the expiration of the patent. In addition, a patent can only be extended once and only for a single product. The U.S. Patent and Trademark Office, in consultation with the FDA, reviews and approves the application for any patent term extension or restoration. In the future, we may intend to apply for restoration of patent term for one of our patents, if and as applicable, to add patent life beyond its current expiration date, depending on the expected length of the clinical trials and other factors involved in the filing of the relevant BLA.
Biosimilars and Reference Product Exclusivity
The ACA includes a subtitle called the Biologics Price Competition and Innovation Act (BPCIA), which created an abbreviated approval pathway for biological products that are biosimilar to or interchangeable with an FDA-approved reference biological product. This amendment to the PHSA attempts to minimize duplicative testing. Biosimilarity, which requires that there be no clinically meaningful differences between the biological product and the reference product in terms of safety, purity, and potency, can be shown through analytical studies, animal studies, and a clinical study or studies. Interchangeability requires that a product is biosimilar to the reference product and the product must demonstrate that it can be expected to produce the same clinical results as the reference product in any given patient and, for products that are administered multiple times to an individual, the biologic and the reference biologic may be alternated or switched after one has been previously administered without increasing safety risks or risks of diminished efficacy relative to exclusive use of the reference biologic.
Under the BPCIA, an application for a biosimilar product may not be submitted to the FDA until four years following the date that the reference product was first licensed by the FDA. In addition, the approval of a biosimilar product may not be made effective by the FDA until 12 years from the date on which the reference product was first licensed. “First licensure” typically means the initial date the particular product at issue was licensed in the United States. This does not include a supplement for the biological product or a subsequent application by the same sponsor or manufacturer of the biological product (or licensor, predecessor in interest, or other related entity) for a change that results in a new indication, route of administration, dosing schedule, dosage form, delivery system, delivery device, or strength, unless that change is a modification to the structure of the biological product and such modification changes its safety, purity, or potency. During this 12-year period of exclusivity, another company may still market a competing version of the reference product if the FDA approves a full BLA for the competing product containing that applicant’s own preclinical data and data from adequate and well-controlled clinical trials to demonstrate the safety, purity and potency of its product. The BPCIA also created certain exclusivity periods for biosimilars approved as interchangeable products.
Foreign Regulation
In order to market any product outside of the United States, we would need to comply with numerous and varying regulatory requirements of other countries and jurisdictions regarding quality, safety, and efficacy and governing, among other things, clinical trials, marketing authorization, commercial sales and distribution of our products. Whether or not we obtain FDA approval for a product, we would need to obtain the necessary approvals by the comparable foreign regulatory authorities before we can commence clinical trials or marketing of the product in foreign countries and jurisdictions. Although many of the issues discussed above with respect to the United States apply similarly in the context of the European Union, the approval process varies between countries and jurisdictions and can involve additional product testing and additional administrative review periods. The time required to obtain approval in other countries and jurisdictions might differ from and be longer than that required to obtain FDA approval. Regulatory approval in one country or jurisdiction does not ensure regulatory approval in another, but a failure or delay in obtaining regulatory approval in one country or jurisdiction may negatively impact the regulatory process in others.
41
Regulation and Procedures Governing Approval of Medicinal Products in Europe
Clinical Trial Approval
In the European Union, our future products also may be subject to extensive regulatory requirements. As in the United States, medicinal products can be marketed only if a marketing authorization from the competent regulatory agencies has been obtained.
Similar to the United States, the various phases of preclinical and clinical research in the European Union are subject to significant regulatory controls.
In April 2014, the EU adopted the new Clinical Trials Regulation (EU) No 536/2014, (Regulation) which replaced the Clinical Trials Directive 2001/20/EC (Directive) on January 31, 2022. The transitory provisions of the Regulation provide that, by January 31, 2025, all ongoing clinical trials must have transitioned to the new Regulation.
The new Regulation is directly applicable in all EU Member States (and so does not require national implementing legislation in each Member State), and aims at simplifying and streamlining the approval of clinical studies in the EU. The main characteristics of the new Regulation include: a streamlined application procedure via a single-entry point through the Clinical Trials Information System, (CTIS); a single set of documents to be prepared and submitted for the application as well as simplified reporting procedures for clinical trial sponsors; and a harmonized procedure for the assessment of applications for clinical trials, which is divided in two parts (Part I contains scientific and medicinal product documentation and Part II contains the national and patient-level documentation). Part I is assessed by a coordinated review by the competent authorities of all EU Member States in which an application for authorization of a clinical trial has been submitted (Concerned Member States) of a draft report prepared by a Reference Member State. Part II is assessed separately by each Concerned Member State. Strict deadlines have also been established for the assessment of clinical trial applications.
Marketing Authorization
To obtain a marketing authorization for a product in the European Union, an applicant must submit a marketing authorization application, either under the centralized procedure administered by the EMA or one of the procedures administered by competent authorities in EU Member States (decentralized procedure, national procedure, or mutual recognition procedure).
The centralized procedure provides for the grant of a single marketing authorization by the European Commission that is valid throughout the EU, and in the additional Member States of the European Economic Area (Iceland, Liechtenstein and Norway), or EEA. Pursuant to Regulation (EC) No. 726/2004, the centralized procedure is compulsory for specific products, including for medicines produced by certain biotechnological processes, products designated as orphan medicinal products, advanced therapy medicinal products (gene therapy, somatic-cell therapy and tissue-engineered products) and products with a new active substance indicated for the treatment of certain diseases, including HIV, AIDS, cancer, neurodegenerative disorders, diabetes, auto-immune and other immune dysfunctions and viral diseases. The centralized procedure is optional for products containing a new active substance not yet authorized in the EU, or for products that constitute a significant therapeutic, scientific or technical innovation or which are in the interests of public health.
Specifically, the grant of a marketing authorization in the EU for products based on genes, tissues or cells, such as gene therapy or somatic-cell therapy medicinal products, is governed in part by Regulation (EC) No 1394/2007 on advanced therapy medicinal products (ATMPs). Regulation (EC) No 1394/2007 lays down specific rules concerning the authorization, supervision, and pharmacovigilance of ATMPs. Manufacturers of ATMPs must demonstrate the quality, safety, and efficacy of their products to the EMA’s Committee for Advanced Therapies, which provides an opinion on the quality, safety and efficacy of each ATMP subject to marketing authorization application which is sent for final approval to the EMA’s Committee for Medicinal Products for Human Use (CHMP). The CHMP recommendation is then sent to the European Commission, which adopts a decision on whether to grant a marketing authorization which is binding in all Member States. Under the centralized procedure, the maximum timeframe for the evaluation of a marketing authorization application is 210 days from receipt of a valid application, excluding clock stops when additional information or written or oral explanation is to be provided by the applicant in response to questions asked by CHMP. Clock stops may extend the timeframe of evaluation of a marketing authorization application considerably beyond 210 days. Accelerated assessment may be granted by the CHMP in exceptional cases, when a medicinal product is expected to be of major public health interest, particularly from the viewpoint of therapeutic innovation. If the CHMP accepts such a request, the timeframe of 210 days for assessment will be reduced to 150 days (excluding clock stops), but it is possible that the CHMP may revert to the standard time limit for the centralized procedure if it determines that the application is no longer appropriate to conduct an accelerated assessment.
Now that the UK (which comprises Great Britain and Northern Ireland) has left the EU, Great Britain will no longer be covered by centralized marketing authorizations (under the Northern Ireland Protocol, centralized marketing authorizations will continue to be recognized in Northern Ireland). All medicinal products with an existing centralized marketing authorization were automatically converted to Great Britain marketing authorizations on January 1, 2021. For a period of three years from January 1, 2021, the Medicines and Healthcare products Regulatory Agency (MHRA), the UK medicines regulator, may rely on a decision taken by the European Commission on the approval of a new marketing authorization in the centralized procedure, in order to more quickly grant a new Great Britain marketing authorization. This is known as the EC Decision Reliance Procedure. On January 24, 2023, the MHRA announced that a new international recognition framework will be put in place from January 1, 2024, which will have regard to decisions on the approval of marketing authorizations made by the EMA and certain other regulators.
42
European Union Data and Marketing Exclusivity
In the EU, innovative medicinal products (including both small molecules and biological medicinal products) approved on the basis of a complete and independent data package, qualify for eight years of data exclusivity upon marketing authorization and an additional two years of market exclusivity. The data exclusivity, if granted, prevents generic or biosimilar applicants from referencing the innovator’s pre-clinical and clinical trial data contained in the dossier of the reference product when applying for a generic or biosimilar marketing authorization in the EU, for a period of eight years from the date on which the reference product was first authorized in the EU. During the additional two-year period of market exclusivity, a generic or biosimilar marketing authorization can be submitted, and the innovator’s data may be referenced, but no generic or biosimilar medicinal product can be placed on the EU market until the expiration of the market exclusivity period. The overall ten-year period will be extended to a maximum of 11 years if, during the first eight years of those ten years, the marketing authorization holder obtains an authorization for one or more new therapeutic indications which, during the scientific evaluation prior to their authorization, are determined to bring a significant clinical benefit in comparison with currently approved therapies. There is no guarantee that a product will be considered by the EMA to be an innovative medicinal product, and products may not qualify for data exclusivity. Even if a product is considered to be an innovative medicinal product so that the innovator gains the prescribed period of data exclusivity, another company could nevertheless also market another version of the product if such company obtained a marketing authorization based on an application with a complete and independent data package of pharmaceutical tests, preclinical tests and clinical trials.
European Union Orphan Designation and Exclusivity
In the EU, the EMA’s Committee for Orphan Medicinal Products grants orphan designation in respect of a product if its sponsor can establish that: (1) the product is intended for the diagnosis, prevention or treatment of a life-threatening or chronically debilitating condition; (2) either (i) such condition affects no more than 5 in 10,000 persons in the EU when the application is made, or (ii) where it is unlikely that the marketing of the medicine, without the benefits derived from orphan status, would generate sufficient return in the EU to justify the necessary investment in its development; and (3)there exists no satisfactory method of diagnosis, prevention or treatment of such condition authorized for marketing in the EU or, if such a method exists, the product would be of a significant benefit to those affected by that condition.
In the EU, orphan designation entitles a party to financial incentives such as reduction of fees or fee waivers, and ten years of market exclusivity is granted following marketing approval for the orphan product. This period may be reduced to six years if, at the end of the fifth year, it is established that the orphan designation criteria are no longer met, including where it is shown that the product is sufficiently profitable not to justify maintenance of market exclusivity. During the period of market exclusivity, a marketing authorization may only be granted to a “similar medicinal product” for the same therapeutic indication if: (i) a second applicant can establish that its product, although similar to the authorized orphan product, is safer, more effective or otherwise clinically superior; (ii) the marketing authorization holder for the authorized orphan product consents to a second orphan medicinal product application; or (iii) the marketing authorization holder for the authorized orphan product cannot supply enough orphan medicinal product. A “similar medicinal product” is defined as a medicinal product containing a similar active substance or substances as contained in an authorized orphan medicinal product, and which is intended for the same therapeutic indication. Orphan designation must be requested before submitting an application for marketing authorization. Orphan designation does not convey any advantage in, or shorten the duration of, the regulatory review and approval process.
Regulatory Requirements After a Marketing Authorization has been Obtained
If an authorization for a medicinal product in the EU is obtained, the holder of the marketing authorization is required to comply with a range of requirements applicable to the manufacturing, marketing, promotion and sale of medicinal products. These include:
The aforementioned EU rules are generally applicable in the EEA.
43
Brexit and the Regulatory Framework in the United Kingdom
The UK formally left the EU on January 31, 2020 and the EU and the UK have concluded a trade and cooperation agreement (the “TCA”), which was provisionally applicable since January 1, 2021 and has been formally applicable since May 1, 2021. The TCA includes specific provisions concerning pharmaceuticals, which include the mutual recognition of GMP, inspections of manufacturing facilities for medicinal products and GMP documents issued, but does not provide for wholesale mutual recognition of UK and EU pharmaceutical regulations. At present, Great Britain has implemented EU legislation on the marketing, promotion and sale of medicinal products through the Human Medicines Regulations 2012 (as amended) (under the Northern Ireland Protocol, the EU regulatory framework continues to apply in Northern Ireland). The regulatory regime in Great Britain therefore aligns in many ways with current EU regulations, however it is possible that these regimes will diverge more significantly in future now that Great Britain’s regulatory system is independent from the EU and the TCA does not provide for mutual recognition of UK and EU pharmaceutical legislation. However, notwithstanding that there is no wholesale recognition of EU pharmaceutical legislation under the TCA, under the new framework mentioned above which will be put in place by the MHRA from January 1, 2024, the MHRA has stated that it will take into account decisions on the approval of marketing authorizations from the EMA (and certain other regulators) when considering an application for a Great Britain marketing authorization. On February 27, 2023, the UK government and the European Commission announced a political agreement in principle to replace the Northern Ireland Protocol with a new set of arrangements, known as the “Windsor Framework”. This new framework fundamentally changes the existing system under the Northern Ireland Protocol, including with respect to the regulation of medicinal products in the UK. In particular, the MHRA will be responsible for approving all medicinal products destined for the UK market (Great Britain and Northern Ireland) and the EMA will no longer have any role in approving medicinal products destined for Northern Ireland. A single UK-wide marketing authorization will be granted by the MHRA for all medicinal products to be sold in the UK, enabling products to be sold in a single pack and under a single authorization throughout the UK. Once the Windsor Framework is approved by the EU-UK Joint Committee, the UK Government and the EU will enact legislative measures to enact it into law.
Pharmaceutical Coverage, Pricing and Reimbursement
Significant uncertainty exists as to the coverage and reimbursement status of any product candidates for which we or our collaborators obtain regulatory approval. In the United States and markets in other countries, sales of any products for which we or our collaborators receive regulatory approval for commercial sale will depend, in part, on the extent to which third-party payors provide coverage, and establish adequate reimbursement levels for such drug products.
Our ability to successfully commercialize our product candidates will depend in part on the extent to which coverage and adequate reimbursement for these products and related treatments will be available from government health administration authorities, private health insurers and other organizations. Even if coverage is provided, the approved reimbursement amount may not be high enough to allow us to establish or maintain pricing sufficient to realize a sufficient return on our investment. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which medications they will pay for and establish reimbursement levels. In the United States, third-party payors include federal and state healthcare programs, government authorities, private managed care providers, private health insurers and other organizations.
Net prices for drugs may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of drugs from countries where they may be sold at lower prices than in the United States. Third-party payors are increasingly challenging the price, examining the medical necessity and reviewing the cost-effectiveness of medical drug products and medical services, in addition to questioning their safety and efficacy. Such payors may limit coverage to specific drug products on an approved list, also known as a formulary, which might not include all of the FDA-approved drugs for a particular indication. We or our collaborators may need to conduct expensive pharmaco-economic studies in order to demonstrate the medical necessity and cost-effectiveness of our products, in addition to the costs required to obtain the FDA approvals. Nonetheless, our product candidates may not be considered medically necessary or cost-effective. Moreover, the process for determining whether a third-party payor will provide coverage for a drug product may be separate from the process for setting the price of a drug product or for establishing the reimbursement rate that such a payor will pay for the drug product. A payor’s decision to provide coverage for a drug product does not imply that an adequate reimbursement rate will be approved. Further, one payor’s determination to provide coverage for a drug product does not assure that other payors will also provide coverage for the drug product. Adequate third-party reimbursement may not be available to enable us to maintain price levels sufficient to realize an appropriate return on our investment in product development.
Different pricing and reimbursement schemes exist in other countries. In the European Union, governments influence the price of pharmaceutical products through their pricing and reimbursement rules and control of national health care systems that fund a large part of the cost of those products to consumers. Some jurisdictions operate positive and negative list systems under which products may only be marketed once a reimbursement price has been agreed. To obtain reimbursement or pricing approval, some of these countries may require the completion of clinical trials that compare the cost-effectiveness of a particular drug candidate to currently available therapies. Other European Union countries allow companies to fix their own prices for medicines, but monitor and control company profits. The downward pressure on health care costs in general, particularly prescription drugs, has become very intense. As a result, increasingly
44
high barriers are being erected to the entry of new products. In addition, in some countries, cross-border imports from low-priced markets exert a commercial pressure on pricing within a country.
The marketability of any product candidates for which we or our collaborators receive regulatory approval for commercial sale may suffer if the government and third-party payors fail to provide adequate coverage and reimbursement. In addition, emphasis on managed care in the United States has increased and we expect will continue to increase the pressure on pharmaceutical pricing. Coverage policies and third-party reimbursement rates may change at any time. Even if favorable coverage and reimbursement status is attained for one or more products for which we or our collaborators receive regulatory approval, less favorable coverage policies and reimbursement rates may be implemented in the future.
Other U.S. Healthcare Laws and Compliance Requirements
Healthcare providers, including physicians, and third-party payors play a primary role in the recommendation and prescription of any product candidates that we may develop for which we obtain marketing approval. Our current and future arrangements with third-party payors, healthcare providers and customers may implicate broadly applicable fraud and abuse and other healthcare laws and regulations. Restrictions under applicable federal and state healthcare laws and regulations, including certain laws and regulations applicable only if we have marketed products, include the following:
45
Some states also impose requirements on manufacturers and distributors to establish the pedigree of product in the chain of distribution, including some states that require manufacturers and others to adopt new technology capable of tracking and tracing product as it moves through the distribution chain. Several states have enacted legislation requiring pharmaceutical companies to establish marketing compliance programs, file periodic reports with the state, make periodic public disclosures on sales, marketing, pricing, track and report gifts, compensation and other remuneration made to physicians and other healthcare providers, clinical trials and other activities, and/or register their sales representatives, as well as to prohibit pharmacies and other healthcare entities from providing certain physician prescribing data to pharmaceutical companies for use in sales and marketing, and to prohibit certain other sales and marketing practices. All of our activities are potentially subject to federal and state consumer protection and unfair competition laws.
The provision of benefits or advantages to physicians to induce or encourage the prescription, recommendation, endorsement, purchase, supply, order, or use of medicinal products is prohibited in the European Union. The provision of benefits or advantages to also induce or reward improper performance generally is sometimes governed by the national anti-bribery laws of European Union Member States, and the Bribery Act 2010 in the United Kingdom. Infringement of these laws could result in substantial fines and imprisonment. EU Directive 2001/83/EC, which is the EU Directive governing medicinal products for human use, further provides that, where medicinal products are being promoted to persons qualified to prescribe or supply them, no gifts, pecuniary advantages or benefits in kind may be supplied, offered or promised to such persons unless they are inexpensive and relevant to the practice of medicine or pharmacy. This provision has been transposed into the Human Medicines Regulations 2012 and so remains applicable in the United Kingdom despite its departure from the EU.
Payments made to physicians in certain European Union Member States must be publicly disclosed. Moreover, agreements with physicians often must be the subject of prior notification and approval by the physician’s employer, his or her competent professional organization, and/or the regulatory authorities of the individual European Union Member States. These requirements are provided in the national laws, industry codes, or professional codes of conduct applicable in the European Union Member States. Failure to comply with these requirements could result in reputational risk, public reprimands, administrative penalties, fines or imprisonment.
Healthcare Reform
In the United States and some foreign jurisdictions, there have been and continue to be ongoing efforts to implement legislative and regulatory changes regarding the healthcare system. A primary trend in the U.S. healthcare industry and elsewhere is cost containment. Government authorities and other third-party payors have attempted to control costs by limiting coverage and the amount of reimbursement for particular medical products and services, implementing reductions in Medicare and other healthcare funding and applying new payment methodologies.
Within the United States, the federal government and individual states have aggressively pursued healthcare reform, as evidenced by the passing of the ACA and the ongoing efforts to modify or repeal that legislation. The ACA substantially changed the way healthcare is financed by both governmental and private insurers and contains a number of provisions that affect coverage and reimbursement of drug products and/or that could potentially reduce the demand for pharmaceutical products such as increasing drug rebates under state Medicaid programs for brand name prescription drugs and extending those rebates to Medicaid managed care and assessing a fee on manufacturers and importers of brand name prescription drugs reimbursed under certain government programs, including Medicare and Medicaid. Modifications have been implemented under the previous presidential administration and additional modifications or repeal may occur.
In addition, other legislative changes have been proposed and adopted in the United States since the ACA was enacted, for example:
46
Furthermore, there has been heightened governmental scrutiny over the manner in which manufacturers set prices for their marketed products, which has resulted in several congressional inquiries and proposed legislation designed to, among other things, bring more transparency to product pricing, review the relationship between pricing and manufacturer patient assistance programs and reform government program reimbursement methodologies for drug products. President Biden has issued multiple executive orders that have sought to reduce prescription drug costs. In addition, in February 2023, HHS issued a proposal in response to an October 2022 executive order from President Biden that includes a proposed prescription drug pricing model that will test whether targeted Medicare payment adjustments will sufficiently incentivize manufacturers to complete confirmatory trials for drugs approved through FDA’s accelerated approval pathway. Although a number of these and other proposed measures may require authorization through additional legislation to become effective, and the Biden administration may reverse or otherwise change these measures, both the Biden administration and Congress have indicated that they will continue to seek new legislative measures to control drug costs.
The Inflation Reduction Act of 2022, or IRA includes several provisions that may impact our business to varying degrees, including provisions that reduce the out-of-pocket spending cap for Medicare Part D beneficiaries from $7,050 to $2,000 starting in 2025, thereby effectively eliminating the coverage gap; impose new manufacturer financial liability on certain drugs under Medicare Part D, allow the U.S. government to negotiate Medicare Part B and Part D price caps for certain high-cost drugs and biologics without generic or biosimilar competition; require companies to pay rebates to Medicare for certain drug prices that increase faster than inflation; and delay until January 1, 2032 the implementation of the HSS rebate rule that would have limited the fees that pharmacy benefit managers can charge. Further, under the IRA, orphan drugs are exempted from the Medicare drug price negotiation program, but only if they have one rare disease designation and for which the only approved indication is for that disease or condition. If a product receives multiple rare disease designations or has multiple approved indications, it may not qualify for the orphan drug exemption. The effects of the IRA on our business and the healthcare industry in general is not yet known.
At the state level, legislatures have also been increasingly passing legislation and implementing regulations designed to control pharmaceutical and biological product pricing, including price or patient reimbursement constraints, discounts, restrictions on certain product access and marketing cost disclosure and transparency measures, and, in some cases, designed to encourage importation from other countries and bulk purchasing.
Employees and Human Capital Resources
As of December 31, 2022, we had 120 full-time employees, including 34 with Ph.D. or M.D. degrees. None of our employees are represented by labor unions or covered by collective bargaining agreements. In February 2023, we announced a reduction-in-force of approximately 50% in connection with a restructuring plan.
We consider our relationship with our employees to be good. Our human capital resources objectives include, as applicable, identifying, recruiting, retaining, incentivizing and integrating our existing and additional employees. The principal purposes of our equity incentive plans are to attract, retain and motivate selected employees, consultants and directors through the granting of stock-based compensation awards.
47
Corporate History and Information
We were incorporated in Ontario, Canada on June 1, 2017, as Longbow Therapeutics Inc. and were reincorporated in the State of Delaware in October 2019. In February 2020, we changed our name to Integral Medicines, Inc. and in August 2020, we changed our name to Graphite Bio, Inc. Research and development of our initial technology ceased at the end of 2018 and we did not have any significant operations or any research and development activities in 2019. We began our current research and development activities and operations in 2020.
Our principal executive office is located at 201 Haskins Way, Suite 210, South San Francisco, CA 94080, and our telephone number is (650) 484-0886. Our website address is https://graphitebio.com/. We do not incorporate the information on or accessible through our website into this Form 10-K, and you should not consider any information on, or that can be accessed through, our website as part of this Form 10-K. We have included our website address in this Form 10-K solely as an inactive textual reference.
We use various trademarks and trade names in our business, including without limitation our corporate name and logo. All other trademarks or trade names referred to in this Form 10-K are the property of their respective owners. Solely for convenience, the trademarks and trade names in this Form 10-K may be referred to without the ® and ™ symbols, but such references should not be construed as any indicator that their respective owners will not assert, to the fullest extent under applicable law, their rights thereto.
Implications of Being an Emerging Growth Company
We qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012, as amended (JOBS Act). As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable generally to public companies. These provisions include, but are not limited to:
We may take advantage of these exemptions until such time that we are no longer an emerging growth company. We will remain an emerging growth company until the earliest to occur of: (i) the last day of the fiscal year in which we have total annual gross revenues of $1.23 billion or more; (ii) December 31, 2026; (iii) the date on which we have issued more than $1.0 billion in nonconvertible debt during the previous three years; or (iv) the last day of the fiscal year in which we are deemed to be a large accelerated filer under the rules of the Securities and Exchange Commission (SEC), which means the market value of our common stock that is held by non-affiliates exceeds $700 million as of the prior June 30th. We may choose to take advantage of some but not all of these exemptions. We have taken advantage of reduced reporting requirements in this Form 10-K. Accordingly, the information contained herein may be different from the information you receive from other public companies in which you hold stock. We have elected to use this extended transition period for complying with new or revised accounting standards that have different effective dates for public and private companies until the earlier of the date we (1) are no longer an emerging growth company or (2) affirmatively and irrevocably opt out of the extended transition period provided in the JOBS Act. As a result, our financial statements may not be comparable to companies that comply with new or revised accounting pronouncements as of public company effective dates.
48
Item 1A. Risk Factors.
This Form 10-K contains forward-looking information based on our current expectations. Because our business is subject to many risks and our actual results may differ materially from any forward-looking statements made by or on behalf of us, this section includes a discussion of important factors that could affect our business, operating results, financial condition and the trading price of our common stock. You should carefully consider these risk factors, together with all of the other information included in this Form 10-K as well as our other publicly available filings with the SEC.
Risks Related to Strategic Alternative Process and Potential Strategic Transaction
We may not be successful in identifying and implementing any strategic business combination or other transaction and any strategic transactions that we may consummate in the future could have negative consequences.
In addition to our efforts, if any, to pursue clinical development of our non-genotoxic conditioning (NGTC) program, we also continue to evaluate all potential strategic options for the company, including a merger, reverse merger, sale, wind-down, liquidation and dissolution or other strategic transaction. However, there can be no assurance that we will be able to successfully consummate any particular strategic transaction. The process of continuing to evaluate these strategic options may be very costly, time-consuming and complex and we have incurred, and we may in the future incur, significant costs related to this continued evaluation, such as legal and accounting fees and expenses and other related charges. We may also incur additional unanticipated expenses in connection with this process. A considerable portion of these costs will be incurred regardless of whether any such course of action is implemented or transaction is completed. Any such expenses will decrease the remaining cash available for use in our business and may diminish or delay any future distributions to our stockholders.
In addition, any strategic business combination or other transactions that we may consummate in the future could have a variety of negative consequences and we may implement a course of action or consummate a transaction that yields unexpected results that adversely affects our business and decreases the remaining cash available for use in our business or the execution of our strategic plan. There can be no assurances that any particular course of action, business arrangement or transaction, or series of transactions, will be pursued, successfully consummated, lead to increased stockholder value, or achieve the anticipated results. Any failure of such potential transaction to achieve the anticipated results could significantly impair our ability to enter into any future strategic transactions and may significantly diminish or delay any future distributions to our stockholders.
We may not realize any additional value in a strategic transaction.
The market capitalization of our company is below the value of our cash and cash equivalents. Potential counterparties in a strategic transaction involving our company may place minimal or no value on our assets given the limited data regarding our lead development program. Further, the development and any potential commercialization of our product candidates will require substantial additional cash to fund the costs associated with conducting the necessary preclinical and clinical testing and obtaining regulatory approval. Consequently, any potential counterparty in a strategic transaction involving our company may choose not to spend additional resources and continue development of our product candidates and may attribute little or no value, in such a transaction, to those product candidates.
If we are successful in completing a strategic transaction, we may be exposed to other operational and financial risks.
Although there can be no assurance that a strategic transaction will result from the process we have undertaken to identify and evaluate strategic alternatives, the negotiation and consummation of any such transaction will require significant time on the part of our management, and the diversion of management’s attention may disrupt our business. The negotiation and consummation of any such transaction may also require more time or greater cash resources than we anticipate and expose us to other operational and financial risks, including:
49
Any of the foregoing risks could have a material adverse effect on our business, financial condition and prospects.
If a strategic transaction is not consummated, our board of directors may decide to pursue a dissolution and liquidation. In such an event, the amount of cash available for distribution to our stockholders will depend heavily on the timing of such liquidation as well as the amount of cash that will need to be reserved for commitments and contingent liabilities.
There can be no assurance that a strategic transaction will be completed. If a strategic transaction is not completed, our board may decide to pursue a dissolution and liquidation. In such an event, the amount of cash available for distribution to our stockholders will depend heavily on the timing of such decision and, as with the passage of time the amount of cash available for distribution will be reduced as we continue to fund our operations. In addition, if our board were to approve and recommend, and our stockholders were to approve, a dissolution and liquidation, we would be required under Delaware corporate law to pay our outstanding obligations, as well as to make reasonable provision for contingent and unknown obligations, prior to making any distributions in liquidation to our stockholders. As a result of this requirement, a portion of our assets may need to be reserved pending the resolution of such obligations and the timing of any such resolution is uncertain. In addition, we may be subject to litigation or other claims related to a dissolution and liquidation. If a dissolution and liquidation were pursued, our board, in consultation with our advisors, would need to evaluate these matters and make a determination about a reasonable amount to reserve. Accordingly, holders of our common stock could lose all or a significant portion of their investment in the event of a liquidation, dissolution or winding up.
Our ability to consummate a strategic transaction depends on our ability to retain our employees required to consummate such transaction.
Our ability to consummate a strategic transaction depends upon our ability to retain our employees required to consummate such a transaction, the loss of whose services may adversely impact the ability to consummate such transaction. In February 2023, we undertook an organizational restructuring that significantly reduced our workforce in order to conserve our capital resources. Our cash conservation activities may yield unintended consequences, such as attrition beyond our planned reduction in workforce and reduced employee morale, which may cause remaining employees to seek alternative employment. Our ability to successfully complete a strategic transaction depends in large part on our ability to retain certain of our remaining personnel. If we are unable to successfully retain our remaining personnel, we are at risk of a disruption to our exploration and consummation of a strategic alternative as well as business operations.
Our corporate restructuring and the associated headcount reduction may not result in anticipated savings, could result in total costs and expenses that are greater than expected, and could disrupt our business.
In February 2023, we undertook an organizational restructuring that significantly reduced our workforce, including the departure of our chief business officer and our chief scientific officer. We may not realize, in full or in part, the anticipated benefits, savings and improvements in our cost structure from our restructuring efforts due to unforeseen difficulties, delays or unexpected costs. If we are unable to realize the expected operational efficiencies and cost savings from the restructuring, our operating results and financial condition would be adversely affected. Furthermore, our restructuring plan may be disruptive to our operations. For example, our headcount reductions could yield unanticipated consequences, such as increased difficulties in implementing our business strategy, including retention of our remaining employees. Employee litigation related to the headcount reduction could be costly and prevent management from fully concentrating on the business.
Any future growth would impose significant added responsibilities on members of management, including the need to identify, recruit, maintain and integrate additional employees. We may not be able to effectively manage our operations or recruit and retain qualified personnel, which may result in weaknesses in our infrastructure and operations, risks that we may not be able to comply with legal and regulatory requirements, and loss of employees and reduced productivity among remaining employees. For example, the workforce reduction may negatively impact our clinical, regulatory, technical operations, and commercial functions, should we choose to continue to pursue them, which would have a negative impact on our ability to successfully develop, and ultimately, commercialize our product candidates. Our future financial performance and our ability to develop our product candidates or additional assets will depend, in part, on our ability to effectively manage any future growth or restructuring, as the case may be. In addition, given the substantial restructuring of our operations, it may be difficult to evaluate our current business and future prospects on the basis of historical operating performance.
The corporate restructuring and reduction in force that we announced in February 2023 may not achieve our intended objectives.
In February 2023, we announced a reduction in force affecting approximately 50% of our workforce to better align our workforce with the needs of our business and focus our capital resources on select product candidates, helping to provide that we are appropriately resourced. This reduction in force may result in unintended consequences and costs, such as the loss of institutional knowledge and expertise, attrition beyond the intended number of employees, decreased morale among our remaining employees, and the risk that we may not achieve the anticipated benefits of the reduction in force. In addition, while certain positions have been eliminated, certain functions necessary to our operations remain, and we may be unsuccessful in distributing the duties and obligations of departed
50
employees among our remaining employees. The reduction in workforce could also make it difficult for us to pursue, or prevent us from pursuing, new opportunities and initiatives due to insufficient personnel, or require us to incur additional and unanticipated costs to hire new personnel to pursue such opportunities or initiatives. If we are unable to realize the anticipated benefits from the reduction in force, or if we experience significant adverse consequences from the reduction in force, our business, financial condition, and results of operations may be materially adversely affected.
We may become involved in securities class action litigation that could divert management’s attention and harm the company’s business, and insurance coverage may not be sufficient to cover all costs and damages.
In the past, securities class action litigation has often followed certain significant business transactions, such as the sale of a company or announcement of any other strategic transaction, or the announcement of negative events, such as negative results from clinical trials. These events may also result in investigations by the SEC. We may be exposed to such litigation or investigation even if no wrongdoing occurred. Litigation and investigations are usually expensive and divert management’s attention and resources, which could adversely affect our business and cash resources and our ability to consummate a potential strategic transaction or the ultimate value our stockholders receive in any such transaction.
Risks Related to Our Financial Position, Limited Operating History and Need for Additional Capital
We have incurred significant losses since our inception, we expect to incur significant losses for the foreseeable future, and we may never achieve or maintain profitability.
Since our inception, we have incurred significant net losses, have not generated any revenue from product sales to date and have financed our operations principally through the net proceeds raised in our initial public offering and private placements of our redeemable convertible preferred stock. Our net loss for the fiscal years ended December 31, 2022 and 2021 was $101.1 and $70.8 million, respectively. As of December 31, 2022, we had an accumulated deficit of $242.4 million. We expect to continue to incur significant and increasing losses for the foreseeable future. The net losses we incur may fluctuate significantly from quarter to quarter and year to year, such that a period-to-period comparison of our results of operations may not be a good indication of our future performance. We anticipate that our expenses will increase substantially if and as we:
To date, we have not successfully completed a clinical trial for any product candidate. We have only had one product candidate in clinical development and expect that it will be many years, if ever, before we have a product candidate ready for commercialization. To become and remain profitable, we must develop and eventually commercialize products with significant market potential. This will require us to be successful in a range of challenging activities, including identifying product candidates, completing preclinical testing and clinical trials of product candidates, obtaining marketing approval for these product candidates, manufacturing, marketing, and selling those products for which we may obtain marketing approval, obtaining market acceptance for such products and satisfying any post-marketing requirements. We may never succeed in these activities and, even if we do, may never generate revenue in an amount sufficient to achieve profitability. All of our programs are currently only in the discovery, preclinical testing or early clinical development
51
stage. We commenced our Phase 1/2 clinical trial of nulabeglogene autogedtemcel (nula-cel), in SCD in November 2021, and in February 2023 announced that we were discontinuing our development of nula-cel. Because of the numerous risks and uncertainties associated with developing gene therapy and gene editing product candidates, we are unable to predict the extent of any future losses or when we will become profitable, if ever. If we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. Our failure to become and remain profitable would decrease the value of our company and our stock price and could impair our ability to raise capital, maintain and fund our research and development efforts, expand our business, or continue our operations. A decline in the value of our company could also cause you to lose all or part of your investment.
Our limited operating history may make it difficult for you to evaluate the performance of our business to date and to assess our future viability.
We are an early-stage company. We were founded in 2017 and commenced operations in 2020. Our operations to date have been limited to organizing and staffing our company, business planning, raising capital, acquiring and developing our platform and technology, identifying potential product candidates, establishing and maintaining our intellectual property portfolio, undertaking preclinical studies and preparing for clinical trials. Other than nula-cel, which was being evaluated in a Phase 1/2 clinical trial, and for which we terminated development of in February 2023, all of our research programs are still in the preclinical or research stage of development, and their risk of failure is high. We have not demonstrated an ability to initiate or successfully complete any clinical trials, including large-scale, pivotal clinical trials, obtain marketing approvals, manufacture a commercial-scale product, or arrange for a third party to do so on our behalf, or conduct sales and marketing activities necessary for successful commercialization. Typically, it takes about 10 to 15 years to develop a new product from the time it is discovered to when it is available for treating patients. Consequently, any predictions you make about the likelihood of our future success or viability may not be as accurate as they could be if we had a longer operating history.
Our limited operating history, particularly in light of the rapidly evolving gene editing field, may make it difficult to evaluate our technology and industry and predict our future performance. Our very short history as an operating company makes any assessment of the likelihood of our future success and viability subject to significant uncertainty. We will encounter risks and difficulties frequently experienced by very early-stage companies in rapidly evolving fields. If we do not address these risks successfully, our business will suffer.
In addition, as a new business, we may encounter other unforeseen expenses, difficulties, complications, delays, and other known and unknown factors. We will need to transition from a company with a research focus to a company capable of supporting commercial activities. We may not be successful in such a transition. If we do not adequately address these risks and difficulties or successfully make such a transition, our business will suffer.
We have never generated revenue from product sales, may never generate any revenue from product sales and may never become profitable.
Our ability to generate revenue from product sales and achieve profitability, if ever, depends on our ability, alone or with collaborative partners, to successfully complete the development of, and obtain the regulatory approvals necessary to commercialize, our current product candidates and any product candidates we may identify for development. We do not anticipate generating revenues from product sales for the next several years, if ever.
Even if one or more of the product candidates we develop are approved for commercial sale, we anticipate incurring significant costs associated with commercializing any approved product candidate. Our expenses could increase beyond expectations if we are required by the U.S. Food and Drug Administration (FDA), the European Medicines Agency (EMA), or other regulatory authorities to perform clinical and other studies in addition to those that we currently anticipate. Even if we are able to generate revenues from the sale of any approved product candidates, we may not become profitable and may need to obtain additional funding to continue operations.
We will need substantial additional funding. If we are unable to raise capital when needed on acceptable terms, or at all, we would be forced to delay, reduce, or terminate our research and product development programs, future commercialization efforts or other operations.
Developing pharmaceutical products, including conducting preclinical studies and clinical trials, is a very time-consuming, expensive and uncertain process that takes years to complete. Our operations have consumed substantial amounts of cash since inception, and we expect our expenses to increase in connection with our ongoing activities, particularly as we identify, continue the research and development of, initiate and conduct clinical trials of, and seek marketing approval for, our product candidates. In addition, if we obtain marketing approval for our product candidates, we expect to incur significant commercialization expenses related to product sales, marketing, manufacturing, and distribution to the extent that such sales, marketing, manufacturing, and distribution are not the responsibility of a collaborator. Other unanticipated costs may also arise. Furthermore, we expect to incur additional costs associated with operating as a public company. Accordingly, we will need to obtain substantial additional funding in connection with our continuing operations. If we are unable to raise capital when needed or on acceptable terms, we would be forced to delay, reduce, or eliminate our research and product development programs, future commercialization efforts or other operations.
52
As of December 31, 2022, our cash and cash equivalents and investments in marketable securities were $283.6 million. We expect that these funds will enable us to fund our operating expenses and capital expenditure requirements into the fourth quarter of 2024. However, our operating plan may change as a result of factors currently unknown to us, and we may need to seek funding sooner than planned. Our future capital requirements will depend on many factors, including:
53
Identifying potential product candidates and conducting preclinical testing and clinical trials is a time-consuming, expensive, and uncertain process that takes years to complete, and we may never generate the necessary data or results required to obtain marketing approval and achieve product sales. In addition, our product candidates, if approved, may not achieve commercial success. Our commercial revenues, if any, will be derived from sales of products that we do not expect to be commercially available for many years, if at all. Accordingly, we will need to continue to rely on additional financing to achieve our business objectives. Adequate additional financing may not be available to us on acceptable terms, or at all. In addition, we may seek additional capital due to favorable market conditions or strategic considerations even if we believe we have sufficient funds for our current or future operating plans.
Any additional fundraising efforts may divert our management from their day-to-day activities, which may adversely affect our ability to develop and commercialize our product candidates. We have no committed sources of additional capital and, if we are unable to raise additional capital in sufficient amounts or on terms acceptable to us, we may have to significantly delay, scale back or discontinue the development or commercialization of our product candidates or other research and development initiatives. Without sufficient funding, our license agreements and any future collaboration agreements may also be terminated if we are unable to meet the payment or other obligations under such agreements.
Until such time, if ever, as we can generate substantial product revenues, we expect to finance our cash needs through a combination of equity offerings, debt financings, collaborations, strategic alliances, and licensing arrangements. To the extent that we raise additional capital through the sale of equity or convertible debt securities, your ownership interest will be diluted, and the terms of these securities may include liquidation or other preferences that adversely affect your rights as a common stockholder. Debt financing, if available, may involve agreements that include covenants limiting or restricting our ability to take specific actions, such as incurring additional debt, making capital expenditures, declaring dividends, and possibly other restrictions.
If we raise funds through additional collaborations, strategic alliances, or licensing arrangements with third parties, we may have to relinquish valuable rights to our technologies, future revenue streams, research programs, or product candidates we develop, or we may have to grant licenses on terms that may not be favorable to us and/or that may reduce the value of our common stock. If we are unable to raise additional funds through equity or debt financings when needed, we may be required to delay, limit, reduce, or terminate our product development or future commercialization efforts or grant rights to develop and market product candidates that we would otherwise prefer to develop and market ourselves.
We may be subject to adverse legislative or regulatory tax changes that could adversely affect our business and financial condition.
The rules dealing with U.S. federal, state and local income taxation are constantly under review by persons involved in the legislative process and by the Internal Revenue Service and the U.S. Treasury Department. Changes to tax laws (which changes may have retroactive application) could adversely affect our stockholders or us. We cannot predict whether, when, in what form, or with what effective dates, tax laws, regulations and rulings may be enacted, promulgated or decided, which could result in an increase in our, or our stockholders’, tax liability or require changes in the manner in which we operate in order to minimize increases in our tax liability.
Our ability to use our U.S. net operating loss carryforwards and certain other U.S. tax attributes may be limited.
As of December 31, 2022 and 2021, we had U.S. federal net operating loss carryforwards of $75.8 and $57.3 million, respectively, (which are not subject to expiration) and state net operating loss carryforwards of $0.6 million (which begin to expire in various amounts in 2039). Our ability to use our U.S. federal and state net operating losses to offset potential future taxable income and reduce income
54
taxes that would otherwise be due is dependent upon our generation of future taxable income, and we cannot predict with certainty when, or whether, we will generate sufficient taxable income to use all of our net operating losses.
Under current law, unused U.S. federal net operating losses generated in taxable years beginning after December 31, 2017 are not subject to expiration and may be carried forward indefinitely. For taxable years beginning after December 31, 2020, however, the deductibility of such U.S. federal net operating losses is limited to 80% of our taxable income in such taxable years. In addition, both our current and our future unused U.S. federal net operating losses and tax credits may be subject to limitation under Sections 382 and 383 of the Internal Revenue Code of 1986, as amended (the “Code”) if we undergo an “ownership change,” generally defined as a greater than 50 percentage point change (by value) in its equity ownership by certain stockholders over a rolling three-year period. We may have experienced such ownership changes in the past, and we may experience ownership changes in the future as a result of our equity offerings or subsequent shifts in our stock ownership, some of which are outside our control. Our net operating losses and tax credits may also be impaired or restricted under state law.
We face risks related to health epidemics, pandemics and other widespread outbreaks of contagious disease, including the ongoing COVID-19 pandemic, which could significantly disrupt our operations, impact our financial results or otherwise adversely impact our business.
Significant outbreaks of contagious diseases, and other adverse public health developments, could have a material impact on our business operations and operating results. The continued spread of COVID-19, including the emergence of subsequent variants like the Omicron variant, which began to spread around the time that our Phase 1/2 clinical trial of nula-cel initially opened for enrollment, has affected segments of the global economy as well as our operations. For example, COVID-19 impacted clinical trial site resourcing, staffing and operations, resulting in longer timeframes than initially anticipated for participant screening and enrollment. In particular, treatment of the first patient in our Phase 1/2 clinical trial of nula-cel was delayed due to screen failure as a result of a prospective participant becoming infected with COVID-19. As a result of the ongoing COVID-19 pandemic or similar public health crises that may arise, we may experience further disruptions that could adversely impact our operations, research and development, including preclinical studies, clinical trials and manufacturing activities, including:
The trading prices for biopharmaceutical companies have been highly volatile as a result of the COVID-19 pandemic, and we may face similar volatility in our stock price.
We cannot predict the scope and severity of any potential business shutdowns or disruptions. If we or any of the third parties with whom we engage were to experience shutdowns or other business disruptions, our ability to conduct our business in the manner and on the timelines presently planned could be materially and negatively affected, which could have a material adverse impact on our business, financial condition, our results of operations and prospects.
Adverse developments affecting the financial services industry, such as actual events or concerns involving liquidity, defaults, or non-performance by financial institutions or transactional counterparties, could adversely affect our current and projected business operations and our financial condition and results of operations.
Actual events involving limited liquidity, defaults, non-performance or other adverse developments that affect financial institutions, transactional counterparties or other companies in the financial services industry or the financial services industry generally,
55
or concerns or rumors about any events of these kinds or other similar risks, have in the past and may in the future lead to market-wide liquidity problems. For example, on March 10, 2023, Silicon Valley Bank (“SVB”) was closed by the California Department of Financial Protection and Innovation, which appointed the Federal Deposit Insurance Corporation (“FDIC”) as receiver. Similarly, on March 12, 2023, Signature Bank and Silvergate Capital Corp. were each swept into receivership. Although a statement by the Department of the Treasury, the Federal Reserve and the FDIC indicated that all depositors of SVB would have access to all of their money after only one business day of closure, including funds held in uninsured deposit accounts, borrowers under credit agreements, letters of credit and certain other financial instruments with SVB, Signature Bank or any other financial institution that is placed into receivership by the FDIC may be unable to access undrawn amounts thereunder.
Less than $1.0 million of our total cash and cash equivalents is held in operating accounts at SVB, representing a de minimus amount of our total cash and cash equivalents. Our deposits with SVB above $250,000 are uninsured. We have letters of credit worth $1.7 million held with SVB related to our leases, which are classified on our balance sheet as restricted cash. Although we are not a borrower or party to any other financial instruments with SVB, Signature or any other financial institution currently in receivership, if any of our lenders or counterparties to any such instruments were to be placed into receivership, we may be unable to access such funds. In addition, if any of our suppliers or other parties with whom we conduct business are unable to access funds pursuant to such instruments or lending arrangements with such a financial institution, such parties’ ability to pay their obligations to us or to enter into new commercial arrangements requiring additional payments to us could be adversely affected. In this regard, counterparties to SVB credit agreements and arrangements, and third parties such as beneficiaries of letters of credit (among others), may experience direct impacts from the closure of SVB and uncertainty remains over liquidity concerns in the broader financial services industry. Similar impacts have occurred in the past, such as during the 2008-2010 financial crisis.
Inflation and rapid increases in interest rates have led to a decline in the trading value of previously issued government securities with interest rates below current market interest rates. Although the U.S. Department of Treasury, FDIC and Federal Reserve Board have announced a program to provide up to $25 billion of loans to financial institutions secured by certain of such government securities held by financial institutions to mitigate the risk of potential losses on the sale of such instruments, widespread demands for customer withdrawals or other liquidity needs of financial institutions for immediately liquidity may exceed the capacity of such program. Additionally, there is no guarantee that the U.S. Department of Treasury, FDIC and Federal Reserve Board will provide access to uninsured funds in the future in the event of the closure of other banks or financial institutions, or that they would do so in a timely fashion.
Although we assess our banking and supplier relationships as we believe necessary or appropriate, our access to funding sources and credit arrangements in amounts adequate to finance or capitalize our current and projected future business operations could be significantly impaired by factors that affect us, the financial institutions with which we may have credit agreements or arrangements directly, or the financial services industry or economy in general. These factors could include, among others, events such as liquidity constraints or failures, the ability to perform obligations under various types of financial, credit or liquidity agreements or arrangements, disruptions or instability in the financial services industry or financial markets, or concerns or negative expectations about the prospects for companies in the financial services industry. These factors could involve financial institutions or financial services industry companies with which we have financial or business relationships, but could also include factors involving financial markets or the financial services industry generally.
The results of events or concerns that involve one or more of these factors could include a variety of material and adverse impacts on our current and projected business operations and our financial condition and results of operations. These could include, but may not be limited to, the following:
In addition, investor concerns regarding the U.S. or international financial systems could result in less favorable commercial financing terms, including higher interest rates or costs and tighter financial and operating covenants, or systemic limitations on access to credit and liquidity sources, thereby making it more difficult for us to acquire financing on acceptable terms or at all. Any decline in available funding or access to our cash and liquidity resources could, among other risks, adversely impact our ability to meet our operating expenses, financial obligations or fulfill our other obligations, result in breaches of our financial and/or contractual obligations or result in violations of federal or state wage and hour laws. Any of these impacts, or any other impacts resulting from the factors described above or other related or similar factors not described above, could have material adverse impacts on our liquidity and our current and/or projected business operations and financial condition and results of operations.
In addition, any further deterioration in the macroeconomic economy or financial services industry could lead to losses or defaults by our suppliers, which in turn, could have a material adverse effect on our current and/or projected business operations and results of
56
operations and financial condition. For example, a supplier may become insolvent or declare bankruptcy, or determine that it will no longer deal with us as a customer. In addition, a supplier could be adversely affected by any of the liquidity or other risks that are described above as factors that could result in material adverse impacts on us, including but not limited to delayed access or loss of access to uninsured deposits or loss of the ability to draw on existing credit facilities involving a troubled or failed financial institution. Any supplier bankruptcy or insolvency, or any breach or default by a supplier, or the loss of any significant supplier relationships, could result in material losses and may have a material adverse impact on our business.
Risks Related to Discovery, Development, and Commercialization
We are very early in our development efforts. All of our product candidates are still in preclinical development or earlier stages and it will be many years before we or our collaborators commercialize a product candidate, if ever. If we are unable to advance our product candidates through preclinical and clinical development, obtain regulatory approval and ultimately commercialize our product candidates, or experience significant delays in doing so, our business will be materially harmed.
We are very early in our development efforts and have focused our research and development efforts to date on gene editing technology, identifying our initial targeted disease indications and our initial product candidates. We have not achieved preclinical proof of concept for the majority of our programs and there is no guarantee that we will achieve it for any of these programs. We may also experience delays in conducting or completing our preclinical studies, including because of supply chain interruptions that could lead to shortages in materials or animals required for such studied. For example, recently it has been reported that there is a shortage of non-human primates for biomedical research, which are used in preclinical studies. To date, we have invested substantially all of our efforts and financial resources in building our gene editing platform, and the identification and preclinical development of our current product candidates. Our ability to generate product revenue, which we do not expect will occur for many years, if ever, will depend heavily on the successful development and eventual commercialization of our product candidates, which may never occur.
Commencing clinical trials in the United States is also subject to acceptance by the FDA of our INDs, and finalizing the trial design based on discussions with the FDA and other regulatory authorities. In the event that the FDA requires us to complete additional preclinical studies or we are required to satisfy other FDA requests, the start of our clinical trials may be delayed. Even after we receive and incorporate guidance from these regulatory authorities, the FDA or other regulatory authorities could disagree that we have satisfied their requirements to commence our clinical trial or change their position on the acceptability of our trial design or the clinical endpoints selected, which may require us to complete additional preclinical studies or clinical trials or impose stricter approval conditions than we currently expect. There are equivalent processes and risks applicable to clinical trial applications in other countries, including in Europe.
Commercialization of our product candidates will require additional preclinical and clinical development; regulatory and marketing approval in multiple jurisdictions, including by the FDA and the EMA; obtaining manufacturing supply, capacity and expertise; building of a commercial organization; and significant marketing efforts. The success of product candidates we identify and develop will depend on many factors, including the following:
57
Additionally, we may be required to change the prioritization of our research and development programs from time to time for a variety of reasons, including our determination that another of our existing or future product candidates has greater potential for clinical development, regulatory approval, or commercialization, including potentially greater therapeutic benefit, a more favorable safety or efficacy profile, a more consistent or more cost effective manufacturing process, or more a favorable commercial profile, including greater market acceptance or commercial potential, or more advantageous intellectual property position. For example, we announced in February 2023 that we were ending our development of nula-cel early, and we currently do not have any ongoing clinical trials. This or any additional shifts in our research and development priorities could result in a delay in clinical development, or we may fail to prioritize programs that may ultimately have the greatest potential for technical or commercial success.
If we do not successfully complete one or more of the activities necessary to achieve regulatory approvals for our product candidates in a timely manner or at all, we could experience significant delays or an inability to successfully commercialize our product candidates, which would materially harm our business. If we do not receive regulatory approvals for our product candidates, we may not be able to continue our operations.
Our gene editing technology is not approved for human therapeutic use. The approaches we are taking to discover and develop novel therapeutics may never lead to marketable products.
We have been focused on developing curative medicines utilizing the CRISPR gene editing technology. CRISPR-based gene editing technologies are relatively new and their therapeutic utility is largely unproven. Our successful development of products will require solving a number of issues, including developing or obtaining technologies to safely deliver a therapeutic agent into target cells within the human body or engineer human cells while outside of the body such that the modified cells can have a therapeutic effect when delivered to the patient, optimizing the efficacy and specificity of such products, and ensuring and demonstrating the therapeutic selectivity, efficacy, potency, purity and safety of such products. There can be no assurance we will be successful in solving any or all of these issues. Indeed, no gene editing cell therapy has been approved in the United States, the European Union (EU), other countries or other key jurisdictions. Accordingly, the potential to successfully obtain approval for any of our CRISPR technology-based therapies remains unproven. We cannot be sure that our gene editing technologies will yield satisfactory products that are safe and effective, sufficiently pure or potent, manufacturable, scalable or profitable in our selected indications.
We are subject to additional development challenges and risks due to the novel nature of our gene editing technology.
Because our technology potentially involves gene editing across multiple cell and tissue types, we are subject to many of the challenges and risks that other gene editing therapeutics and gene therapies face, including:
Further, because our ex vivo product candidates involve editing human cells and then delivering modified cells to patients, we are subject to many of the challenges and risks that engineered cell therapies face. For example, clinical trials using engineered cell-based gene therapies may require unique products to be created for each patient and such individualistic manufacturing may be both inefficient and cost-prohibitive.
We may not be successful in our efforts to identify and develop potential product candidates. If these efforts are unsuccessful, we may never become a commercial stage company or generate any revenues.
The success of our business depends primarily upon our ability to identify, develop, and commercialize product candidates based on our gene editing platform. Our research programs may fail to identify potential product candidates for clinical development for a number of reasons: our research methodology may be unsuccessful in identifying potential product candidates; our potential product candidates may be shown to have harmful side effects in preclinical in vitro experiments or animal model studies; they may not show
58
promising signals of therapeutic effect in such experiments or studies; or they may have other characteristics that may make the product candidates impractical to manufacture, unmarketable, or unlikely to receive marketing approval.
If any of these events occur, we may be forced to abandon our research or development efforts for a program or programs, which would have a material adverse effect on our business, financial condition, results of operations, and prospects and could potentially cause us to cease operations. Research programs to identify new product candidates require substantial technical, financial, and human resources. We may focus our efforts and resources on potential programs or product candidates that ultimately prove to be unsuccessful, which would be costly and time-consuming.
If serious adverse events, undesirable side effects, or unexpected characteristics are identified with respect to our product candidates, we may need to abandon or limit our clinical development or commercialization of those product candidates.
To date, we have not completed the evaluation of any product candidates in human clinical trials. It is impossible to predict when or if any product candidates we develop will ultimately prove safe in humans. In the genomic medicine field, there have been several significant adverse events from gene therapy treatments in the past, including reported cases of leukemia and death. There can be no assurance that our gene editing technologies will not cause severe or undesirable side effects. For example, in January 2023, we announced that the first patient dosed with nula-cel had experienced a serious and unexpected adverse event of prolonged low blood cell counts (pancytopenia) requiring ongoing transfusion and growth factor support, following which we discontinued further development of nula-cel for sickle cell disease.
59
A significant potential risk in any gene editing product is that the edit will be “off-target” and cause serious adverse events, undesirable side effects, or unexpected characteristics. For example, off-target cuts could lead to disruption of a gene or a genetic regulatory sequence at an unintended site in the DNA. We cannot be certain that off-target editing will not occur in any of our clinical studies. There is also the potential risk of delayed adverse events following exposure to gene editing therapy due to the potential for persistent biological activity of the genetic material or other components of products used to carry the genetic material. If any adverse events or side effects are caused by any product candidate we develop and test, the administration process or related procedures, our clinical trials could be delayed, suspended or terminated.
Viral vectors, including AAV, which are relatively new approaches used for disease treatment, also have known side effects, and for which additional risks could develop in the future. In past clinical trials that were conducted by others with certain viral vectors, significant side effects were caused by gene therapy treatments, including reported cases of myelodysplasia, leukemia and death. Other potential side effects could include an immunologic reaction and insertional oncogenesis, which is the process whereby the insertion of a functional gene near a gene that is important in cell growth or division results in uncontrolled cell division, which could potentially enhance the risk of cancer. If the vectors we use demonstrate a similar side effect, or other adverse events, we may be required to halt or delay further clinical development of any potential product candidates.
Furthermore, the FDA has stated that lentiviral vectors, which are used in certain gene integration approaches, possess characteristics that may pose high risks of delayed adverse events. Such delayed adverse events may also occur in other viral vectors, including AAV vectors. In addition to side effects and adverse events caused by our product candidates, the conditioning, administration process or related procedures which may be used to condition a patient for gene therapy treatment also can cause adverse side effects and adverse events. A gene therapy patient is generally administered cytotoxic drugs to remove stem cells from the bone marrow to create sufficient space in the bone marrow for the modified stem cells to engraft and produce new cells. This procedure compromises the patient’s immune system, and conditioning regimens have been associated with adverse events in clinical trial participants.
If any other product candidates we develop are associated with serious adverse events, undesirable side effects, or unexpected characteristics, we may need to abandon their development or limit development to certain uses or subpopulations in which the serious adverse events, undesirable side effects or other characteristics are less prevalent, less severe, or more acceptable from a risk-benefit perspective, any of which would have a material adverse effect on our business, financial condition, results of operations, and prospects. Many product candidates, including gene therapy product candidates, that initially showed promise in early-stage testing have later been found to cause side effects that prevented further clinical development of the product candidates.
If we are unable to demonstrate that any of the above adverse events were caused by factors other than our product candidate, the FDA, the EMA or other regulatory authorities could order us to cease further development of, or deny approval of, any product candidates we are able to develop for any or all targeted indications. Even if we are able to demonstrate that all future serious adverse events are not product-related, such occurrences could affect patient recruitment. Moreover, if we elect, or are required, to delay, suspend or terminate any clinical trial of any product candidate we may develop, the commercial prospects of such product candidates may be harmed and our ability to generate product revenues from any of these product candidates may be delayed or eliminated. Any of these occurrences may harm our ability to identify and develop product candidates and could have a material adverse effect on our business, financial condition, result of operations, and prospects.
Additionally, if we successfully develop a product candidate and it receives marketing approval, the FDA could require us to adopt a Risk Evaluation and Mitigation Strategy (REMS), to ensure that the benefits of treatment with such product candidate outweighs the risks for each potential patient, which may include, among other things, a medication guide outlining the risks of the product for distribution to patients, a communication plan to health care practitioners, extensive patient monitoring, or distribution systems and processes that are highly controlled, restrictive, and more costly than what is typical for the industry. Furthermore, if we or others later identify undesirable side effects caused by any product candidate that we develop, several potentially significant negative consequences could result, including:
Any of these events could prevent us from achieving or maintaining market acceptance of any of our product candidates and could have a material adverse effect on our business, financial condition, results of operations, and prospects.
60
We may find it difficult to enroll patients in clinical trials given the limited number of patients who have the diseases for which our product candidates may be developed. If we experience delays or difficulties in the enrollment of patients in clinical trials, our clinical development activities and our receipt of necessary regulatory approvals could be delayed or prevented.
We may not be able to initiate or continue clinical trials for any product candidates we identify or develop if we are unable to locate and enroll a sufficient number of eligible patients to participate in these trials as required by the FDA, the EMA or other analogous regulatory authorities outside the United States, or as needed to provide appropriate statistical power for a given trial. Enrollment may be particularly challenging for some of the rare genetically defined diseases targeted in our most advanced programs. In addition, if patients are unwilling to participate in our gene editing trials because of negative publicity from adverse events related to the biotechnology, gene therapy, or gene editing fields, negative publicity related to our discontinued clinical program, competitive clinical trials for similar patient populations, clinical trials in competing products, or for other reasons, the timeline for recruiting patients, conducting studies, and obtaining regulatory approval of our product candidates may be delayed. Moreover, some of our competitors may have ongoing clinical trials for product candidates that would treat the same indications as our product candidates, and patients who would otherwise be eligible for our clinical trials may instead enroll in clinical trials of our competitors’ product candidates.
Patient enrollment is also affected by other factors, including:
In addition, the ongoing COVID-19 pandemic has affected, and may continue to affect, the timing of our planned clinical trials. For instance, the treatment of the first patient in our Phase 1/2 clinical trial was delayed due to a prospective participant becoming infected with COVID-19. We may continue to be adversely affected by the COVID-19 pandemic. As the global healthcare community responded to the fluctuations in COVID-19 cases and hospitalizations, many hospitals, including those operating as clinical trial sites for other ongoing trials, temporarily paused elective procedures, which included dosing of new patients with investigational products. Additionally, the COVID-19 pandemic may cause delays in data collection and monitoring activities, which may present data integrity challenges or require modifications to our planned clinical trial protocols.
In addition, our ability to successfully initiate, enroll, and complete a clinical trial in any foreign country is subject to numerous risks unique to conducting business in foreign countries, including:
61
Enrollment delays in our clinical trials may result in increased development costs for our product candidates, which would cause the value of our Company to decline and limit our ability to obtain additional financing. If we or our collaborators have difficulty enrolling a sufficient number of patients to conduct our clinical trials as planned, we may need to delay, limit, or terminate ongoing or planned clinical trials, any of which would have an adverse effect on our business, financial condition, results of operations, and prospects.
62
If we are unable to successfully identify patients who are likely to benefit from therapy with our product candidates, or experience significant delays in doing so, we may not realize the full commercial potential of any products we may develop.
Our success may depend, in part, on our ability to identify patients who are likely to benefit from therapy with our product candidates, which requires those potential patients to have their DNA analyzed for the presence or absence of a particular sequence. If we, or any third parties that we engage to assist us, are unable to successfully identify such patients, or experience delays in doing so, then:
As a result of these factors, we may be unable to successfully develop and realize the commercial potential of our product candidates, and our business, financial condition, results of operations, and prospects would be materially adversely affected.
Even if we complete the necessary preclinical studies and clinical trials, the marketing approval process is expensive, time-consuming, and uncertain and may prevent us from obtaining approvals for the commercialization of our product candidates. If we are not able to obtain, or if there are delays in obtaining, required regulatory approvals, we will not be able to commercialize, or will be delayed in commercializing, product candidates we develop, and our ability to generate revenue will be materially impaired.
Any product candidates we develop and the activities associated with their development and commercialization, including their design, testing, manufacture, recordkeeping, labeling, storage, approval, advertising, promotion, sale, import, export, and distribution, are subject to comprehensive regulation by the FDA, the EMA and other regulatory authorities in the United States and by comparable authorities in other countries. Failure to obtain marketing approval for a product candidate will prevent us from commercializing the product candidate in a given jurisdiction. We have not received approval to market any product candidates from regulatory authorities in any jurisdiction. We have only limited experience in filing and supporting the applications necessary to gain marketing approvals and expect to rely on third parties to assist us in this process. Securing regulatory approval requires the submission of extensive preclinical and clinical data and supporting information to the various regulatory authorities for each therapeutic indication to establish the biological product candidate’s safety, purity, and potency. Securing regulatory approval also requires the submission of extensive information about the product manufacturing process, and inspection of manufacturing facilities by, the relevant regulatory authority. Our product candidates may not be effective, may be only moderately effective, or may prove to have undesirable or unintended side effects, toxicities, or other characteristics that may preclude our obtaining marketing approval or prevent or limit commercial use.
The process of obtaining marketing approvals, both in the United States and abroad, is expensive, may take many years if approval is obtained at all, and can vary substantially based upon a variety of factors, including the type, complexity, and novelty of the product candidates involved. Changes in marketing approval policies during the development period, changes in or the enactment of additional statutes or regulations, or changes in regulatory review for each submitted product application, may cause delays in the approval or rejection of an application. The FDA and comparable authorities in other countries have substantial discretion in the approval process and may refuse to accept any application or may decide that our data is insufficient for approval and require additional preclinical, clinical, or other studies. In addition, varying interpretations of the data obtained from preclinical and clinical testing could delay, limit, or prevent marketing approval of a product candidate. Any marketing approval we ultimately obtain may be limited or subject to restrictions or post-approval commitments that render the approved product not commercially viable.
If we experience delays in obtaining approval or if we fail to obtain approval of our product candidates, the commercial prospects for those product candidates may be harmed, and our ability to generate revenues will be materially impaired.
We may expend our limited resources to pursue a particular product candidate or indication and fail to capitalize on product candidates or indications that may be more profitable or for which there is a greater likelihood of success.
Because we have limited financial and managerial resources, we focus on research programs and product candidates that we identify for specific indications among many potential options. As a result, we may forego or delay pursuit of opportunities with other product candidates or for other indications that later prove to have greater commercial potential. For example, we plan to evaluate our NGTC program in additional preclinical studies instead of advancing our other product candidate, nula-cel, as we believe this approach has the potential to provide more value to our shareholders. Our resource allocation decisions may cause us to fail to capitalize on viable commercial products or profitable market opportunities. Our spending on current and future research and development programs and product candidates for specific indications may not yield any commercially viable products. If we do not accurately evaluate the commercial potential or target market for a particular product candidate, we may relinquish valuable rights to that product candidate through collaboration, licensing, or other royalty arrangements in cases in which it would have been more advantageous for us to retain sole development and commercialization rights to such product candidate. Any such event could have a material adverse effect on our business, financial condition, results of operations, and prospects.
63
Even if we complete the necessary clinical trials, we cannot predict when, or if, we will obtain regulatory approval to commercialize a product candidate we may develop in the United States or any other jurisdiction, and any such approval may be for a more narrow indication than we seek.
We cannot commercialize a product candidate until the appropriate regulatory authorities have reviewed and approved the product candidate. Even if our product candidates meet their safety and efficacy endpoints in clinical trials, the regulatory authorities may not complete their review processes in a timely manner, or we may not be able to obtain regulatory approval. Additional delays may result if an FDA Advisory Committee or other regulatory authority recommends non-approval or restrictions on approval. In addition, we may experience delays or rejections based upon additional government regulation from future legislation or administrative action, or changes in regulatory authority policy during the period of product development, clinical trials, and the review process.
Regulatory authorities also may approve a product candidate for more limited indications than requested or they may impose significant limitations in the form of narrow indications, warnings or a REMS. These regulatory authorities may require labeling that includes precautions or contraindications with respect to conditions of use, or they may grant approval subject to the performance of costly post-marketing clinical trials. In addition, regulatory authorities may not approve the labeling claims that are necessary or desirable for the successful commercialization of our product candidates. Any of the foregoing scenarios could materially harm the commercial prospects for our product candidates and materially adversely affect our business, financial condition, results of operations, and prospects.
Marketing approval by the FDA in the United States, if obtained, does not ensure approval by regulatory authorities in other countries or jurisdictions. In addition, clinical trials conducted in one country may not be accepted by regulatory authorities in other countries, and regulatory approval in one country does not guarantee regulatory approval in any other country. Approval processes vary among countries and can involve additional product candidate testing and validation and additional administrative review periods. Seeking regulatory approval outside the United States could result in difficulties and costs for us and require additional preclinical studies or clinical trials which could be costly and time-consuming. Regulatory requirements can vary widely from country to country and could delay or prevent the introduction of our product candidates in those countries. The foreign regulatory approval process involves all of the risks associated with FDA approval. We do not have any product candidates approved for sale in any jurisdiction, including international markets, and we do not have experience in obtaining regulatory approval in international markets. If we fail to comply with regulatory requirements in international markets or to obtain and maintain required approvals, or if regulatory approvals in international markets are delayed, our target market will be reduced and our ability to realize the full market potential of our product candidates will be unrealized.
Our product candidates may fail to achieve the degree of market acceptance by physicians, patients, third-party payors, and others in the medical community necessary for commercial success.
The commercial success of any of our product candidates will depend upon its degree of market acceptance by physicians, patients, third-party payors, and others in the medical community. Ethical, social, and legal concerns about genetic medicines generally and gene editing technologies specifically could result in additional regulations restricting or prohibiting the marketing of our product candidates. Even if our product candidates receive marketing approval, they may nonetheless fail to gain sufficient market acceptance by physicians, patients, third-party payors, and others in the medical community. The degree of market acceptance of our product candidates, if approved for commercial sale, will depend on a number of factors, including:
64
Even if any of our product candidates obtain regulatory approval, such products may not achieve an adequate level of acceptance, we may not generate or derive sufficient product revenues, and we may not become profitable.
We face significant competition in an environment of rapid technological change, and there is a possibility that our competitors may achieve regulatory approval before us or develop therapies that are safer, less expensive or more advanced or effective than ours, which may harm our financial condition and our ability to successfully market or commercialize our product candidates.
The development and commercialization of new drug products is highly competitive. Moreover, the gene editing field is characterized by rapidly changing technologies, significant competition, and a strong emphasis on intellectual property. We will face competition with respect to any product candidates that we may develop or commercialize in the future from major pharmaceutical companies, specialty pharmaceutical companies, and biotechnology companies worldwide. Potential competitors also include academic institutions, government agencies, and other public and private research organizations that conduct research, seek patent protection, and establish collaborative arrangements for research, development, manufacturing, and commercialization.
There are a number of large pharmaceutical and biotechnology companies that currently market and sell products or are pursuing the development of products for the treatment of the disease indications for which we have research programs. Some of these competitive products and therapies are based on scientific approaches that are the same as or similar to our approach, and others are based on entirely different approaches.
There are several other companies advancing gene editing and gene editing and gene therapy product candidates in preclinical or clinical development in sickle cell disease, including Beam Therapeutics Inc., bluebird bio, Inc., Cellectis SA, CRISPR Therapeutics AG, Editas Medicine, Inc., Intellia Therapeutics, Inc., and Sangamo Therapeutics, Inc. Companies advancing gene therapy programs in beta-thalassemia include bluebird bio, Inc., CRISPR Therapeutics AG, Sangamo Therapeutics, Inc. and Edigene Inc. Companies advancing gene therapy programs in XSCID include Mustang Bio, Inc. Companies advancing gene therapy programs in Gaucher Disease include AVROBio, Inc. and Freeline Therapeutics Holdings plc. Companies advancing gene editing and gene therapy programs in preclinical development for AAT deficiency include Beam Therapeutics Inc., Editas Medicine, Inc., Intellia Therapeutics, Inc., Krystal Biotech Inc., Apic Bio Inc., and LogicBio Therapeutics Inc. Companies combining CRISPR with HDR (homology directed repair) include CRISPR Therapeutics AG, which, for oncology applications, inserts a chimeric antigen receptor (CAR) construct into the TCR alpha constant (TRAC) locus in T-cells using HDR. Additionally, an academic collaboration between the University of California, San Francisco and the University of California, Los Angeles is seeking to correct the sickle cell mutation using CRISPR followed by delivery of a single-stranded oligonucleotide DNA donor to potentially harness HDR.
Any product candidates that we successfully develop and commercialize will compete with existing therapies and new therapies that may become available in the future that are approved to treat the same diseases for which we may obtain approval for our product candidates. This may include other types of therapies, such as small molecule, antibody, and/or protein therapies.
Many of our current or potential competitors, either alone or with their collaboration partners, may have significantly greater financial resources and expertise than we do in research and development, manufacturing, preclinical testing, conducting clinical trials, obtaining regulatory approvals, and marketing approved products. Mergers and acquisitions in the pharmaceutical, biotechnology, and gene therapy industries may result in even more resources being concentrated among a smaller number of our competitors. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with large and established companies. These competitors also compete with us in recruiting and retaining qualified scientific and management personnel and establishing clinical trial sites and patient registration for clinical trials, as well as in acquiring technologies complementary to, or necessary for, our programs. Our commercial opportunity could be reduced or eliminated if our competitors develop and commercialize product candidates that are safer, more effective, have fewer or less severe side effects, are more convenient, or are less expensive than any product candidates that we may develop or that would render any product candidates that we may develop obsolete or non-competitive. Our competitors also may obtain FDA or other regulatory approval for their product candidates more rapidly than we may obtain approval for ours, which could result in our competitors establishing a strong market position before we are able to enter the market. Additionally, technologies developed by our competitors may render our potential product candidates uneconomical or obsolete, and we may not be successful in marketing our product candidates against competitors.
65
In addition, as a result of the expiration or successful challenge of our patent rights, we could face more litigation with respect to the validity and/or scope of patents relating to our competitors’ products. The availability of our competitors’ products could limit the demand, and the price we are able to charge, for any product candidates that we may develop and commercialize.
If, in the future, we are unable to establish sales and marketing capabilities or enter into agreements with third parties to sell and market our product candidates, we may not be successful in commercializing those product candidates if and when they are approved.
We do not have a sales or marketing infrastructure and have limited experience in the sale, marketing, or distribution of pharmaceutical products. To achieve commercial success for any approved products for which we retain sales and marketing responsibilities, we must either develop a sales and marketing organization or outsource these functions to third parties. In the future, we may choose to build a focused sales, marketing, and commercial support infrastructure to sell, or participate in sales activities with our collaborators for, some of our product candidates if and when they are approved.
There are risks involved with both establishing our own commercial capabilities and entering into arrangements with third parties to perform these services. For example, recruiting and training a sales force or reimbursement specialists is expensive and time consuming and could delay any product launch. If the commercial launch of a product candidate for which we recruit a sales force and establish marketing and other commercialization capabilities is delayed or does not occur for any reason, we would have prematurely or unnecessarily incurred these commercialization expenses. This may be costly, and our investment would be lost if we cannot retain or reposition our commercialization personnel.
Factors that may inhibit our efforts to commercialize our product candidates on our own include:
If we enter into arrangements with third parties to perform sales, marketing, commercial support, and distribution services, our product revenues or the profitability of these product revenues to us may be lower than if we were to market and sell any products we may develop ourselves. In addition, we may not be successful in entering into arrangements with third parties to commercialize our product candidates or may be unable to do so on terms that are favorable to us. We may have little control over such third parties, and any of them may fail to devote the necessary resources and attention to sell and market our products effectively. If we do not establish commercialization capabilities successfully, either on our own or in collaboration with third parties, we will not be successful in commercializing our product candidates.
Adverse public perception of genetic medicines and gene editing in particular, may negatively impact regulatory approval of, and/or demand for, our potential products, if approved.
Our potential therapeutic products involve editing the human genome. The clinical and commercial success of our potential products will depend in part on public understanding and acceptance of the use of gene editing therapy for the prevention or treatment of human diseases. Public perception and related media coverage of potential gene therapy-related efficacy or safety issues, including adoption of new therapeutics or novel approaches to treatment, as well as ethical concerns related specifically to gene editing, may adversely influence the willingness of subjects to participate in clinical trials, or if any therapeutic is approved, of physicians and patients to accept these novel and personalized treatments. Physicians, health care providers and third-party payors often are slow to adopt new products, technologies and treatment practices, particularly those that may also require additional upfront costs and training. Physicians may not be willing to undergo training to adopt these novel and potentially personalized therapies, may decide the particular therapy is too complex or potentially risky to adopt without appropriate training, and may choose not to administer the therapy. Further, due to health conditions, genetic profile or other reasons, certain patients may not be candidates for the therapies.
In addition, responses by federal and state agencies, congressional committees and foreign governments to negative public perception, ethical concerns or financial considerations may result in new legislation, regulations, or medical standards, such as stricter labeling requirements, that could limit our ability to develop or commercialize any product candidates, obtain or maintain regulatory
66
approval or otherwise achieve profitability. New government requirements may be established that could delay or prevent regulatory approval of our product candidates under development. It is impossible to predict whether legislative changes will be enacted, regulations, policies or guidance changed, or interpretations by agencies or courts changed, or what the impact of such changes, if any, may be. Based on these and other factors, health care providers and payors may decide that the benefits of these new therapies do not or will not outweigh their costs.
More restrictive government regulations or negative public opinion would have a negative effect on our business or financial condition and may delay or impair our development and commercialization of product candidates or demand for our product candidates. Adverse events in our preclinical studies or clinical trials or those of our competitors or of academic researchers utilizing gene editing technologies, even if not ultimately attributable to product candidates we identify and develop, and the resulting publicity could result in increased governmental regulation, unfavorable public perception, potential regulatory delays in the testing or approval of potential product candidates we identify and develop, stricter labeling requirements for those product candidates that are approved, and a decrease in demand for any such product candidates. Use of gene editing technology by a third party or government to develop biological agents or products that threaten U.S. national security could similarly result in such negative impacts to us.
Due to the novel nature of our technology and the potential for our product candidates to offer therapeutic benefit in a single administration or limited number of administrations, we face uncertainty related to pricing and reimbursement for these product candidates. Likewise, even if we are able to commercialize any product candidates, such products may become subject to unfavorable pricing regulations, third-party reimbursement practices, or healthcare reform initiatives, which would harm our business.
The regulations that govern marketing approvals, pricing, and reimbursement for new products vary widely from country to country. Some countries require approval of the sale price of a product before it can be marketed. In many countries, the pricing review period begins after marketing or product licensing approval is granted. In some foreign markets, prescription pharmaceutical pricing remains subject to continuing governmental control even after initial approval is granted. As a result, we might obtain marketing approval for a product in a particular country, but then be subject to price regulations that delay or might even prevent our commercial launch of the product, possibly for lengthy time periods, and negatively impact the revenues we are able to generate from the sale of the product in that country. Adverse pricing limitations may hinder our ability to recoup our investment in one or more product candidates we develop, even if any of our product candidates obtain marketing approval. See section entitled “Business – Government Regulation - Pharmaceutical Coverage, Pricing and Reimbursement.”
In the United States, no uniform policy exists for coverage and reimbursement for products among third-party payors. Therefore, decisions regarding the extent of coverage and amount of reimbursement to be provided can differ significantly from payor to payor. The process for determining whether a payor will provide coverage for a product may be separate from the process for setting the reimbursement rate a payor will pay for the product. One third-party payor’s decision to cover a particular product or service does not ensure that other payors will also provide coverage for the medical product or service. Third-party payors may limit coverage to specific products on an approved list or formulary, which may not include all FDA-approved products for a particular indication.
We expect the cost of a single administration of a gene editing therapy, such as those we have been seeking to develop, to be substantial, when and if they achieve regulatory approval. Coverage and reimbursement by government and private payors will be essential for most patients to be able to afford these treatments. Accordingly, sales of any such product candidates will depend substantially, both domestically and abroad, on the extent to which the costs of any of our product candidates will be paid by government authorities, private health plans, and other third-party payors. Payors may not be willing to pay high prices for a single administration. Coverage and reimbursement by a third-party payor may depend upon several factors, including the third-party payor’s determination that use of a product is:
We cannot be sure that reimbursement will be available for any products that we commercialize and, if reimbursement is available, that the level of reimbursement will be adequate. Reimbursement may impact the demand for, or the price of, any product candidate for which we obtain marketing approval. If reimbursement is not available or is available only to limited levels, we may not be able to successfully commercialize any product candidate for which we obtain marketing approval. Obtaining coverage and reimbursement for a product from third-party payors is a time-consuming and costly process that could require us to provide to the payor supporting scientific, clinical, and cost-effectiveness data. There is significant uncertainty related to third-party coverage and reimbursement of newly approved products. We may not be able to provide data sufficient to gain acceptance with respect to coverage and reimbursement. If coverage and reimbursement are not available, or are available only at limited levels, we may not be able to successfully commercialize any of our product candidates. Even if coverage is provided, the approved reimbursement amount may not be adequate to realize a sufficient return on our investment.
67
Our initial target patient populations are relatively small, as a result of which the pricing and reimbursement of any of our product candidates, if approved, must be adequate to support the necessary commercial infrastructure. If we are unable to obtain adequate levels of reimbursement, our ability to successfully market and sell any such product candidates will be adversely affected. The manner and level at which reimbursement is provided for services related to any product candidates we develop (e.g., for administration of our product candidate to patients) is also important. Inadequate reimbursement for such services may lead to physician and payor resistance and adversely affect our ability to market or sell our product candidates. In addition, we may need to develop new reimbursement models in order to realize adequate value. Payors may not be able or willing to adopt such new models, and patients may be unable to afford that portion of the cost that such models may require them to bear. If we determine such new models are necessary but we are unsuccessful in developing them, or if such models are not adopted by payors, our business, financial condition, results of operations, and prospects could be adversely affected.
There may be significant delays in obtaining reimbursement for newly approved products, and coverage may be more limited than the purposes for which the product is approved by the FDA, the EMA or other regulatory authorities outside the United States. Moreover, eligibility for reimbursement does not imply that any product will be paid for in all cases or at a rate that covers our costs, including research, development, manufacture, sale, and distribution. Interim reimbursement levels for new products, if applicable, may also not be sufficient to cover our costs and may not be made permanent. Reimbursement rates may vary according to the use of the product and the clinical setting in which it is used, may be based on reimbursement levels already set for lower cost products and may be incorporated into existing payments for other services. Net prices for products may be reduced by mandatory discounts or rebates required by government healthcare programs or private payors and by any future relaxation of laws that presently restrict imports of products from countries where they may be sold at lower prices than in the United States. Our inability to promptly obtain coverage and profitable payment rates from both government-funded and private payors for any approved products we may develop could have a material adverse effect on our operating results, our ability to raise capital needed to commercialize products, and our overall financial condition.
If the market opportunities for any product candidates we develop are smaller than we believe they are, our potential revenues may be adversely affected, and our business may suffer. Because the target patient populations for many of our product candidates are small, we must be able to successfully identify patients and achieve a significant market share to maintain profitability and growth.
We focus our research and product development on treatments for rare genetically defined diseases. Many of our product candidates are expected to target a single mutation; as a result, the relevant patient population may therefore be small. Our projections of both the number of people who have these diseases, as well as the subset of people with these diseases who have the potential to benefit from treatment with our product candidates, are based on estimates. These estimates may prove to be incorrect and new studies may change the estimated incidence or prevalence of these diseases. The number of patients in the United States, Europe, and elsewhere may turn out to be lower than expected, and patients may not be amenable to treatment with our product candidates, or may become increasingly difficult to identify or gain access to, all of which would adversely affect our business, financial condition, results of operations, and prospects. Additionally, because of the potential that any product candidates we develop could cure a target disease, we may not receive recurring revenues from patients and may deplete the patient population prevalence through curative therapy.
Genetic medicines are novel, and any product candidates we develop may be complex and difficult to manufacture. We could experience delays in complying with regulatory requirements or production problems that result in delays in our development or commercialization programs, limit the supply of our product candidates, or otherwise harm our business.
Our product candidates will likely require processing steps that are more complex than those required for most chemical pharmaceuticals. Moreover, unlike chemical pharmaceuticals, the physical and chemical properties of a biologic such as the product candidates we are developing generally cannot be fully characterized. As a result, assays of the finished product candidate may not be sufficient to ensure that the product candidate will perform in the intended manner. Problems with the manufacturing process, even minor deviations from the normal process, could result in product defects or manufacturing failures that result in unusable products, product recalls, product liability claims, insufficient inventory, or potentially delay progression of our potential IND filings. We may also encounter problems achieving adequate quantities and quality of clinical-grade materials that meet FDA, EMA or other comparable applicable foreign standards or specifications with consistent and acceptable production yields and costs. For example, the current approach of manufacturing AAV vectors may fall short of supplying required number of doses needed for advanced stages of pre-clinical studies or clinical trials, and the FDA may ask us to demonstrate that we have the appropriate manufacturing processes in place to support the higher-dose group in our future pre-clinical studies or clinical trials.
In addition, the FDA, the EMA, and other regulatory authorities may require us to submit samples of any of the approved product together with the protocols showing the results of applicable tests at any time. Under some circumstances, the FDA, the EMA, or other regulatory authorities may require that we not distribute a sample until the agency authorizes its release. Slight deviations in the manufacturing process, including those affecting quality attributes and stability, may result in unacceptable changes in the product that
68
could result in product recalls. Product recalls could cause us to delay clinical trials or product launches, which could be costly to us and otherwise harm our business, financial condition, results of operations, and prospects.
We also may encounter problems hiring and retaining the experienced scientific, quality control, and manufacturing personnel needed to manage our manufacturing process, which could result in delays in our production or difficulties in maintaining compliance with applicable regulatory requirements.
Given the nature of biologics manufacturing, including for AAV vectors, there is a risk of contamination during manufacturing. Any contamination could materially harm our ability to produce product candidates on schedule and could harm our results of operations and cause reputational damage. Some of the raw materials that we anticipate will be required in our manufacturing process are derived from biologic sources. Such raw materials are difficult to procure and may be subject to contamination or recall. A material shortage, contamination, recall, or restriction on the use of biologically derived substances in the manufacture of our current or future product candidates could adversely impact or disrupt the commercial manufacturing or the production of clinical material, which could materially harm our development timelines and our business, financial condition, results of operations, and prospects.
Any problems in our manufacturing process or the facilities with which we contract could make us a less attractive collaborator for potential partners, including larger pharmaceutical companies and academic research institutions, which could limit our access to additional attractive development programs. We have partnered with an experienced manufacturer, WuXi, to support the testing, development and manufacturing of our product candidates, but, problems in third-party manufacturing process or facilities also could restrict our ability to ensure sufficient clinical material for our planned clinical trials and to meet market demand for any product candidates we develop and commercialize.
Product liability lawsuits against us could cause us to incur substantial liabilities and could limit commercialization of our product candidates.
We face an inherent risk of product liability exposure related to the testing in human clinical trials of our product candidates and will face an even greater risk if we commercially sell any products we develop. If we cannot successfully defend ourselves against claims that our product candidates caused injuries, we could incur substantial liabilities. Regardless of merit or eventual outcome, liability claims may result in:
Although we maintain product liability insurance coverage, it may not be adequate to cover all liabilities that we may incur. We anticipate that we will need to increase our insurance coverage when we begin clinical trials and if we successfully commercialize any products. Insurance coverage is increasingly expensive. We may not be able to maintain insurance coverage at a reasonable cost or in an amount adequate to satisfy any liability that may arise.
If we or any contract manufacturers and suppliers we engage fail to comply with environmental, health, and safety laws and regulations, we could become subject to fines or penalties or incur costs that could have a material adverse effect on the success of our business.
We and any contract manufacturers and suppliers we engage are subject to numerous federal, state, and local environmental, health, and safety laws, regulations, and permitting requirements, including those governing laboratory procedures; the generation, handling, use, storage, treatment, and disposal of hazardous and regulated materials and wastes; the emission and discharge of hazardous materials into the ground, air, and water; and employee health and safety. Our operations involve the use of hazardous and flammable materials, including chemicals and biological and radioactive materials. Our operations also produce hazardous waste. We generally contract with third parties for the disposal of these materials and wastes. We cannot eliminate the risk of contamination or injury from these materials. In the event of contamination or injury resulting from our use of hazardous materials, we could be held liable for any resulting damages, and any liability could exceed our resources. Under certain environmental laws, we could be held responsible for costs relating to any contamination at our current or past facilities and at third-party facilities. We also could incur significant costs associated with civil or criminal fines and penalties.
Compliance with applicable environmental laws and regulations may be expensive, and current or future environmental laws and regulations may impair our research and product development efforts. In addition, we cannot entirely eliminate the risk of accidental injury or contamination from these materials or wastes. Although we maintain workers’ compensation insurance to cover us for costs
69
and expenses we may incur due to injuries to our employees resulting from the use of hazardous materials, this insurance may not provide adequate coverage against potential liabilities. We do not carry specific biological or hazardous waste insurance coverage, and our property, casualty, and general liability insurance policies specifically exclude coverage for damages and fines arising from biological or hazardous waste exposure or contamination. Accordingly, in the event of contamination or injury, we could be held liable for damages or be penalized with fines in an amount exceeding our resources, and our clinical trials or regulatory approvals could be suspended, which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
In addition, we may incur substantial costs in order to comply with current or future environmental, health, and safety laws, regulations, and permitting requirements. These current or future laws, regulations, and permitting requirements may impair our research, development, or production efforts. Failure to comply with these laws, regulations, and permitting requirements also may result in substantial fines, penalties, or other sanctions or business disruption, which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
Any third-party contract manufacturers and suppliers we engage will also be subject to these and other environmental, health, and safety laws and regulations. Liabilities they incur pursuant to these laws and regulations could result in significant costs or an interruption in operations, which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
Risks Related to Our Relationships with Third Parties
We expect to rely on third parties to conduct our clinical trials and some aspects of our research and preclinical testing, and those third parties may not perform satisfactorily, including failing to meet deadlines for the completion of such trials, research, or testing.
We currently rely and expect to continue to rely on third parties, such as CROs, clinical data management organizations, medical institutions, and clinical investigators, to conduct some aspects of our research and preclinical testing, and we expect to rely on third parties to help conduct our planned clinical trials. Any of these third parties may terminate their engagements with us at any time under certain criteria. If we need to enter into alternative arrangements, it may delay our product development activities.
Our reliance on these third parties to conduct clinical trials and for research and development activities will reduce our control over these activities but will not relieve us of our responsibilities. For example, we will remain responsible for ensuring that each of our clinical trials is conducted in accordance with the general investigational plan and protocols for the trial. Moreover, the FDA, the EMA and other regulatory authorities require us to comply with standards, commonly referred to as Good Clinical Practices, for conducting, recording, and reporting the results of clinical trials to assure that data and reported results are credible and accurate and that the rights, integrity, and confidentiality of trial participants are protected.
Although we intend to design the clinical trials for future product candidates, CROs will conduct some or all of the clinical trials. As a result, many important aspects of our development programs, including their conduct and timing, will be outside of our direct control. Our reliance on third parties to conduct current and future preclinical studies and future clinical trials will also result in less direct control over the management of data developed through preclinical studies and clinical trials than would be the case if we were relying entirely upon our own staff. Communicating with third parties can also be challenging, potentially leading to mistakes as well as difficulties in coordinating activities. Third parties may:
These factors may materially adversely affect the willingness or ability of third parties to conduct our preclinical studies and clinical trials and may subject us to unexpected cost increases that are beyond our control. If the CROs and other third parties do not perform preclinical studies and future clinical trials in a satisfactory manner, breach their obligations to us or fail to comply with regulatory requirements, the development, regulatory approval and commercialization of our product candidates may be delayed, we may not be able to obtain regulatory approval and commercialize our product candidates, or our development programs may be materially and irreversibly harmed. If we are unable to rely on preclinical and clinical data collected by our CROs and other third parties, we could be required to repeat, extend the duration of, or increase the size of any preclinical studies or clinical trials we conduct and this could significantly delay commercialization and require greater expenditures, which could have a material adverse effect on our business, financial condition, result of operations, and prospects.
We also expect to rely on third parties to store and distribute drug supplies for our clinical trials. Any performance failure on the part of our distributors could delay clinical development or marketing approval of our product candidates or commercialization of our therapies, producing additional losses and depriving us of potential product revenue.
70
Dr. Matthew Porteus, our co-founder and a member of our board of directors, may have actual or potential conflicts of interest because of his position with Stanford.
Dr. Porteus serves on our board of directors, our Scientific & Clinical Advisory Board and as our paid consultant and retains his position and affiliation with Stanford. Furthermore, Dr. Porteus holds shares of our restricted common stock subject to vesting based, among other things, on his continued service to us as a director, employee or consultant. Dr. Porteus’ position at Stanford creates, or may create the appearance of, conflicts of interest when we ask Dr. Porteus to make decisions that could have different implications for Stanford than the decisions have for us or for himself, including decisions related to our license of intellectual property rights from Stanford and other contractual relationships we may enter into from time to time with Stanford.
We contract with third parties for the manufacture of materials for our research programs and preclinical studies and expect to continue to do so for clinical trials and for commercialization of our product candidates. This reliance on third parties increases the risk that we will not have sufficient quantities of such materials, product candidates, or any products that we may develop and commercialize, or that such supply will not be available to us at an acceptable cost or timelines, which could delay, prevent, or impair our development or commercialization efforts.
We do not have any manufacturing facilities at the present time. We currently rely on third-party manufacturers for the manufacture of materials for our research programs and preclinical studies, including our viral vectors, GMP plasmids, RNA guides and Cas9, and expect to continue to rely on third parties, including Stanford and WuXi, for our planned clinical trials and for commercialization of any product candidates for which we obtain marketing approval. For example, we rely on third parties to manufacture viral vectors. We do not have a long-term supply agreement with any of our third-party manufacturers other than WuXi, and we purchase our required supply on a purchase order basis, which means that aside from any binding purchase orders we have from time to time, our third-party manufacturers could cease manufacturing for us or change the terms on which they are willing to continue manufacturing for us at any time.
We may be unable to establish any agreements with third-party manufacturers or to do so on acceptable terms for one or more of our material needs. Even if we are able to establish agreements with third-party manufacturers, reliance on third-party manufacturers entails additional risks, including:
Third-party manufacturers may not be able to comply with cGMP regulations or similar regulatory requirements outside the United States. Our failure, or the failure of our third-party manufacturers, to comply with applicable regulations could result in sanctions being imposed on us, including fines, injunctions, civil penalties, delays, suspension or withdrawal of approvals, license revocations, seizures or recalls of product candidates, operating restrictions, and criminal prosecutions, any of which could significantly and adversely affect supplies of our products and harm our business, financial condition, results of operations, and prospects.
Any products that we may develop may compete with other product candidates and products for access to manufacturing facilities. There are a limited number of suppliers or manufacturers that operate under cGMP regulations and that might be capable of manufacturing for us.
Any performance failure on the part of our existing or future manufacturers could delay clinical development or marketing approval. We do not currently have arrangements in place for redundant supply for bulk drug substances. If any one of our current contract manufacturer cannot perform as agreed, we may be required to replace that manufacturer, or we may be forced to manufacture the materials ourselves, for which we may not have the capabilities or resources, or enter into an agreement with a different third-party manufacturer, which we may not be able to do on reasonable terms, if at all. In either scenario, our clinical trials supply could be delayed significantly as we establish alternative supply sources. In some cases, the technical skills required to manufacture our products or product candidates may be unique or proprietary to the original third-party manufacturer and we may have difficulty, or there may be contractual restrictions prohibiting us from, transferring such skills to a back-up or alternate supplier, or we may be unable to transfer such skills at all. In addition, if we are required to change third-party manufacturers for any reason, we will be required to verify that the new third-party manufacturer maintains facilities and procedures that comply with quality standards and with all applicable regulations. We will also need to verify, such as through a manufacturing comparability study, that any new manufacturing process will produce our product candidate according to the specifications previously submitted to the FDA or another regulatory authority. The delays associated with the verification of a new third- party manufacturer could negatively affect our ability to develop product
71
candidates or commercialize our products in a timely manner or within budget. Furthermore, a third-party manufacturer may possess technology related to the manufacture of our product candidate that such third-party manufacturer owns independently. This would increase our reliance on such third-party manufacturer or require us to obtain a license from such third- party manufacturer in order to have another third-party manufacturer manufacture our product candidates. In addition, changes in manufacturers often involve changes in manufacturing procedures and processes, which could require that we conduct bridging studies between our prior clinical supply used in our clinical trials and that of any new manufacturer. We may be unsuccessful in demonstrating the comparability of clinical supplies which could require the conduct of additional clinical trials.
Our current and anticipated future dependence upon others for the manufacture of our product candidates or any products may adversely affect our future profit margins and our ability to commercialize any products that receive marketing approval on a timely and competitive basis.
We may enter into collaborations with third parties for the research, development, and commercialization of certain of the product candidates we may develop. If any such collaborations are not successful, we may not be able to capitalize on the market potential of those product candidates.
We may seek third-party collaborators for the research, development, and commercialization of certain of the product candidates we may develop. If we enter into any such arrangements with any third parties, we will likely have limited control over the amount and timing of resources that our collaborators dedicate to the development or commercialization of any product candidates we may seek to develop with them. Our ability to generate revenues from these arrangements will depend on our collaborators’ abilities to successfully perform the functions assigned to them in these arrangements. We cannot predict the success of any collaboration that we enter into.
72
If our collaborations do not result in the successful development and commercialization of product candidates, or if one of our collaborators terminates its agreement with us, we may not receive any future research funding or milestone or royalty payments under the collaboration. If we do not receive the funding we expect under these agreements, our development of product candidates could be delayed, and we may need additional resources to develop product candidates. In addition, if one of our collaborators terminates its agreement with us, we may find it more difficult to find a suitable replacement collaborator or attract new collaborators, and our development programs may be delayed or the perception of us in the business and financial communities could be adversely affected. All of the risks relating to product development, regulatory approval, and commercialization described here apply to the activities of our collaborators.
If conflicts arise between us and our collaborators or strategic partners, these parties may act in a manner adverse to us and could limit our ability to implement our strategies.
If conflicts arise between our corporate or academic collaborators or strategic partners and us, the other party may act in a manner adverse to us and could limit our ability to implement our strategies. Some of our academic collaborators and strategic partners are conducting multiple product development efforts within each area that is the subject of the collaboration with us. Our collaborators or strategic partners, however, may develop, either alone or with others, products in related fields that are competitive with the product candidates we develop that are the subject of these collaborations with us. Competing products, either developed by the collaborators or strategic partners or to which the collaborators or strategic partners have rights, may result in the withdrawal of partner support for our product candidates.
Some of our collaborators or strategic partners could also become our competitors in the future. Our collaborators or strategic partners could develop competing products, preclude us from entering into collaborations with their competitors, fail to obtain timely regulatory approvals, terminate their agreements with us prematurely, or fail to devote sufficient resources to the development and commercialization of products. Any of these developments could harm our product development efforts.
If we are not able to establish collaborations on a timely basis, on commercially reasonable terms, or at all, we may have to alter, reduce or delay our development and commercialization plans, or increase our expenditures to fund development or commercialization activities at our own expense.
For some of the product candidates we may develop, we may decide to collaborate with other pharmaceutical and biotechnology companies for the development and potential commercialization of those product candidates. We face significant competition in seeking appropriate collaborations and collaborations are complex and time-consuming to negotiate and document. Whether we reach a definitive agreement for a collaboration will depend, among other things, upon our assessment of the collaborator’s resources and expertise, the terms and conditions of the proposed collaboration, and the proposed collaborator’s evaluation of a number of factors. Those factors may include the design or results of clinical trials, the likelihood of approval by the FDA, the EMA or similar regulatory authorities outside the United States, the potential market for the subject product candidate, the costs and complexities of manufacturing and delivering such product candidate to patients, the potential of competing products, the existence of uncertainty with respect to our ownership of technology, which can exist if there is a challenge to such ownership without regard to the merits of the challenge, and industry and market conditions generally. The collaborator may also consider alternative product candidates or technologies for similar indications that may be available to collaborate on and whether such a collaboration could be more attractive than the one with us.
We may also be restricted under existing collaboration agreements from entering into future collaboration agreements on certain terms with potential collaborators. In addition, there have been a significant number of recent business combinations among large pharmaceutical companies that have resulted in a reduced number of potential future collaborators, which further increases competition we face in seeking potential collaborations.
We may not be able to negotiate collaborations on a timely basis, on acceptable terms, or at all. If we are unable to do so, we may have to curtail the development of the product candidate for which we are seeking to collaborate, reduce or delay its development program or one or more of our other development programs, delay its potential commercialization or reduce the scope of any sales or marketing activities, or increase our expenditures and undertake development or commercialization activities at our own expense. If we elect to increase our expenditures to fund development or commercialization activities on our own, we may need to obtain additional capital, which may not be available to us on acceptable terms or at all. If we do not have sufficient funds, we may not be able to develop product candidates or bring them to market and generate product revenue.
Risks Related to Our Intellectual Property
If we are unable to obtain and maintain patent and other intellectual property protection for any product candidates we develop and for our platform technology, or if the scope of the patent and other intellectual property protection obtained is not sufficiently broad, our competitors could develop and commercialize products and technology similar or identical to ours, and our ability to successfully commercialize our product candidates, and our platform technology may be adversely affected.
Our commercial success will depend in large part on our ability to obtain and maintain patent, trademark, trade secret and other intellectual property protection of our platform technology, product candidates and other technology, methods used to manufacture them
73
and methods of treatment, as well as successfully defending our patent and other intellectual property rights against third-party challenges. It is difficult and costly to protect our platform technology and product candidates, and we may not be able to ensure their protection. Our ability to stop unauthorized third parties from making, using, selling, offering to sell, importing or otherwise commercializing our product candidates is dependent upon the extent to which we have rights under valid and enforceable patents or trade secrets that cover these activities.
We seek to protect our proprietary position by in-licensing intellectual property relating to our platform technology and filing patent applications in the United States and abroad related to our platform technology and product candidates that are important to our business. If we or our licensors are unable to obtain or maintain patent protection with respect to our platform technology and our product candidates, or if the scope of the patent protection secured is not sufficiently broad, our competitors could develop and commercialize products and technology similar or identical to ours and our ability to commercialize our product candidates may be adversely affected.
The patent prosecution process is expensive, time-consuming, and complex, and we may not be able to file, prosecute, maintain, enforce, or license all necessary or desirable patent applications at a reasonable cost or in a timely manner. In addition, we may not pursue or obtain patent protection in all relevant markets. It is also possible that we will fail to identify patentable aspects of our research and development output in time to obtain patent protection. Although we enter into non-disclosure and confidentiality agreements with parties who have access to confidential or patentable aspects of our research and development output, such as our employees, corporate collaborators, outside scientific collaborators, CROs, contract manufacturers, consultants, advisors, and other third parties, any of these parties may breach the agreements and disclose such output before a patent application is filed, thereby jeopardizing our ability to seek patent protection. In addition, our ability to obtain and maintain valid and enforceable patents depends on whether the differences between our inventions and the prior art allow our inventions to be patentable over the prior art. Furthermore, publications of discoveries in the scientific literature often lag behind the actual discoveries, and patent applications in the United States and other jurisdictions are typically not published until 18 months after filing, or in some cases not at all. Therefore, we cannot be certain that we or our licensors were the first to make the inventions claimed in our owned or any licensed patents or pending patent applications, or that we or our licensors were the first to file for patent protection of such inventions.
The patent position of biotechnology and pharmaceutical companies generally is highly uncertain, involves complex legal and factual questions, and has been the subject of much litigation in recent years. The field of gene editing has been the subject of extensive patenting activity and litigation. As a result, the issuance, scope, validity, enforceability, and commercial value of our patent rights are highly uncertain and we may become involved in complex and costly litigation. Our pending and future patent applications may not result in patents being issued which protect our platform technology and our product candidates or which effectively prevent others from commercializing competitive technologies and product candidates.
No consistent policy regarding the scope of claims allowable in the field of gene editing has emerged in the United States. The scope of patent protection outside of the United States is also uncertain. Changes in either the patent laws or their interpretation in the United States and other countries may diminish our ability to protect our inventions, obtain, maintain, enforce and defend our intellectual property rights and, more generally, could affect the value of our intellectual property or narrow the scope of our owned and licensed patent rights. With respect to both in-licensed and owned intellectual property, we cannot predict whether the patent applications we and our licensors are currently pursuing will issue as patents in any particular jurisdiction or whether the claims of any issued patents will be valid and enforceable and provide sufficient protection from competitors. Further, it is anticipated that in mid-2023, European patent applications will have the option, upon grant of a patent, of becoming a Unitary Patent which will be subject to the jurisdiction of the Unitary Patent Court ("UPC"). This will be a significant change in European patent practice. As the UPC is a new court system, there is no precedent for the court, increasing the uncertainty of any litigation.
Moreover, the coverage claimed in a patent application can be significantly reduced before the patent is issued, and its scope can be reinterpreted after issuance. Even if patent applications we license or own currently or in the future issue as patents, they may not issue in a form that will provide us with any meaningful protection, prevent competitors or other third parties from competing with us, or otherwise provide us with any competitive advantage. Any patents that we own or in-license may be challenged, narrowed, circumvented, or invalidated by third parties. Consequently, we do not know whether any of our platform advances and product candidates will be protectable or remain protected by valid and enforceable patents. Our competitors or other third parties may be able to circumvent our patents by developing similar or alternative technologies or products in a non-infringing manner.
In addition, given the amount of time required for the development, testing, and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, our intellectual property may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours. Moreover, some of our owned and in-licensed patents and patent applications may in the future be, co-owned by us with third parties. If we are unable to obtain an exclusive license to such third-party co-owners’ interest in such patents or patent applications, such co-owners may be able to license their rights to other third parties, including our competitors, and our competitors could market competing products and technology. In addition, we may need the cooperation of any such co-owners of our patent rights in order to enforce such
74
patents against third parties, and such cooperation may not be provided to us. Any of the foregoing could have a material adverse effect on our competitive position, business, financial conditions, results of operations, and prospects.
Our rights to develop and commercialize our gene editing platform technology and product candidates are subject, in part, to the terms and conditions of licenses granted to us by others.
We depend on intellectual property licensed from third parties, and our licensors may not always act in our best interest. If we fail to comply with our obligations under our intellectual property licenses, if the licenses are terminated, or if disputes regarding these licenses arise, we could lose significant rights that are important to our business.
We have licensed and are dependent on certain patent rights and proprietary technology from third parties that are important or necessary to the development of our gene editing technology and product candidates. For example, we are a party to a license agreement with Stanford pursuant to which we in-license key patent applications for our gene editing platform technology and product candidates (the Stanford License Agreement). This license agreement imposes various diligence, milestone payment, royalty, insurance, and other obligations on us. If we fail to comply with these obligations, our licensors may have the right to terminate our license, in which event we would not be able to develop or market our gene editing platform or any other technology or product candidates covered by the intellectual property licensed under this agreement. For example, under the Stanford License Agreement, we are required to initiate clinical trial programs in accordance with the development plan and development milestones for the development of a licensed product covered by the licensed patent rights. If we fail to initiate such clinical trial programs, our rights with respect to the licensed patent rights may terminate.
These and other licenses may not provide exclusive rights to use such intellectual property and technology in all relevant fields of use and in all territories in which we may wish to develop or commercialize our gene editing platform technology and product candidates in the future. Some licenses granted to us are expressly subject to certain preexisting rights held by the licensors or certain third parties. As a result, we may not be able to prevent competitors from developing and commercializing competitive products in certain territories or fields. For example, the Stanford License Agreement provides that our field of use is solely for the development of prophylactics and therapeutics for sickle cell disease, XSCID, and beta-thalassemia. If we determine that rights to such additional fields are necessary to commercialize our product candidates or maintain our competitive advantage, we may will need to obtain a license from Stanford and/or other third parties in order to continue developing, manufacturing or marketing our product candidates. We may not be able to obtain such a license on an exclusive basis, on commercially reasonable terms, or at all, which could prevent us from commercializing our product candidates or allow our competitors or others the chance to access technology that is important to our business.
We do not have complete control in the preparation, filing, prosecution, and maintenance of the patent applications covering the technology that we license from third parties. For example, pursuant to our intellectual property license with Stanford, our licensors retain control of preparation, filing, prosecution, and maintenance of their patent applications. We cannot be certain that these patent applications will be prepared, filed, prosecuted, maintained, and defended in a manner consistent with the best interests of our business. If our licensors fail to prosecute, and maintain such patent applications, or lose rights to those patent applications, the rights we have licensed may be reduced or eliminated, our right to develop and commercialize any of our product candidates that are the subject of such licensed rights could be adversely affected and we may not be able to prevent competitors from making, using, and selling competing products.
Certain of our licensors have also relied on third-party collaborators or on funds from third parties such that they are not the sole and exclusive owners of the patent rights we have in-licensed. For example, our in-licensed patent rights from the Stanford License are jointly owned by Stanford and Agilent Technologies, Inc. (Agilent). We may be required to incur additional costs in connection with obtaining licenses to the rights of Agilent, and if we are unable to secure such licenses, the license granted to us in jurisdictions where the consent of a co-owner is necessary to grant such a license may not be valid. As a result, Agilent could license such patent rights to our competitors, and our competitors could market competing products and technology. In addition, our rights to our in-licensed patent applications are dependent, in part, on inter-institutional or other operating agreements between Stanford and Agilent. If Stanford or Agilent breaches such inter-institutional or operating agreements, our rights to such in-licensed patents and patent applications may be adversely affected. Any of these events could have a material adverse effect on our competitive position, business, financial condition, results of operations, and prospects.
Furthermore, inventions contained within some of our in-licensed patent applications were made using U.S. government funding. We rely on our licensors to ensure compliance with applicable obligations arising from such funding, such as timely reporting, an obligation associated with our in-licensed patents and patent applications. The failure of our licensors to meet their obligations may lead to a loss of rights or the unenforceability of relevant patents. For example, the U.S. government could have certain rights in such in-licensed patent applications, including a non-exclusive license authorizing the U.S. government to use the invention or to have others use the invention on its behalf. If the U.S. government decides to exercise these rights, it is not required to engage us as its contractor in connection with doing so. The U.S. government’s rights may also permit it to disclose the funded inventions and technology to third parties and to exercise march-in rights to use or allow third parties to use the technology we have licensed that was developed using U.S. government funding. The U.S. government may also exercise its march-in rights if it determines that action is necessary because we or our licensors failed to achieve practical application of the U.S. government-funded technology, because action is necessary to
75
alleviate health or safety needs, to meet requirements of federal regulations, or to give preference to U.S. industry. In addition, our rights in such in-licensed U.S. government-funded inventions may be subject to certain requirements to manufacture product candidates embodying such inventions in the United States. Any of the foregoing could harm our business, financial condition, results of operations, and prospects significantly.
In the event that any of our third-party licensors determines that, in spite of our efforts, we have materially breached a license agreement or have failed to meet certain obligations thereunder, it may elect to terminate the license agreement or, in some cases, one or more license(s) under the applicable license agreement and such termination would result in us no longer having the ability to develop and commercialize product candidates and technology covered by that license agreement. In the event of such termination of a third-party in-license, or if the underlying patent rights under a third-party in-license fail to provide the intended exclusivity, competitors would have the freedom to seek regulatory approval of, and to market, products identical to ours. Any of these events could have a material adverse effect on our competitive position, business, financial conditions, results of operations, and prospects of our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection but where enforcement is not as strong as that in the United States. These products may compete with our product candidates and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets, and other intellectual property protection, particularly those relating to biotechnology and pharmaceutical products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products against third parties in violation of our intellectual property and proprietary rights generally. Proceedings to enforce our patents and intellectual property rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing, and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. Moreover, the initiation of proceedings by third parties to challenge the scope or validity of our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business. Accordingly, our efforts to enforce our intellectual property and proprietary rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Many countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If we or our licensors is forced to grant one or more licenses to third parties with respect to any patents relevant to our business, our competitive position may be impaired, and our business, financial condition, results of operations, and prospects may be adversely affected.
Our owned and in-licensed patent applications may not provide sufficient protection of our platform technologies, our product candidates and our future product candidates or result in any competitive advantage.
We own and have in-licensed a number of patent applications that cover gene editing, gene targeting and NGTC technologies. We have applied for provisional patent applications intended to specifically cover our platform technology and uses with respect to treatment of particular diseases and conditions, but do not currently own any issued U.S. patents. Each U.S. provisional patent application is not eligible to become an issued patent until, among other things, we file a non-provisional patent application within 12 months of the filing date of the applicable provisional patent application. Any failure to file a non-provisional patent application within this timeline could cause us to lose the ability to obtain patent protection for the intentions disclosed in the associated provisional patent applications. We cannot be certain that any of these patent applications will issue as patents, and if they do, that such patents will cover or adequately protect our gene editing platform technologies or our product candidates, or that such patents will not be challenged, narrowed, circumvented, invalidated or held unenforceable. Any failure to obtain or maintain patent protection with respect to our platform technology and product candidates could have a material adverse effect on our business, financial condition, results of operations and growth prospects.
Our owned and in-licensed patent applications contain claims directed to compositions of matter on our gene editing and NGTC product candidates, as well as methods directed to the therapeutic use of such product candidates. Method-of-use patents do not prevent a competitor or other third party from developing or marketing an identical product for an indication that is outside the scope of the patented method. Moreover, with respect to method-of-use patents, even if competitors or other third parties do not actively promote their product for our targeted indications or uses for which we may obtain patents, providers may recommend that patients use these products off-label, or patients may do so themselves.
The strength of patents in the biotechnology and pharmaceutical field involves complex legal and scientific questions and can be uncertain. The patent applications that we own or in-license may fail to result in issued patents with claims that cover our product candidates or uses thereof in the United States or in other foreign countries. For example, while our patent applications are pending, we
76
may be subject to a third party pre-issuance submission of prior art to the United States Patent and Trademark Office (USPTO), or become involved in interference or derivation proceedings, or equivalent proceedings in foreign jurisdictions. Even if patents do successfully issue, third parties may challenge their inventorship, validity, enforceability or scope, including through opposition, revocation, reexamination, post-grant and inter partes review proceedings. An adverse determination in any such submission, proceeding or litigation could reduce the scope of, or invalidate or render unenforceable, our owned or in-licensed patent rights, allow third parties to commercialize our technology or product candidates and compete directly with us, without payment to us, or result in our inability to manufacture or commercialize products without infringing third-party patent rights. Moreover, we, or our licensor, may have to participate in interference proceedings declared by the USPTO to determine priority of invention or in post-grant challenge proceedings, such as oppositions in a foreign patent office, that challenge our or our licensor’s priority of invention or other features of patentability with respect to our owned or in-licensed patent applications. For example, the European Patent Office (EPO) Opposition Division has initiated opposition proceedings against an in-licensed European patent. Such challenges, including this current opposition, may result in loss of patent rights, loss pof exclusivity, or in patent claims being narrowed, invalidated, or held unenforceable, which could limit our ability to stop others from using or commercializing similar or identical technology and products, or limit the duration of the patent protection of our technology and product candidates. Furthermore, even if they are unchallenged, our patent rights may not adequately protect our intellectual property or prevent others from designing around our platform technology or product candidates. If the breadth or strength of protection provided by the patent applications we own or in-license with respect to our platform technology and product candidates is threatened, it could dissuade companies from collaborating with us to develop, and threaten our ability to commercialize, our product candidates. Further, if we encounter delays in development, testing, and regulatory review of new product candidates, the period of time during which we could market our product candidates under patent protection would be reduced.
Given that patent applications in the United States and other countries are confidential for a period of time after filing, at any moment in time, we cannot be certain that we or our licensors were in the past or will be in the future the first to file any patent application related to our gene editing and NGTC technology or product candidates. In addition, some patent applications in the United States may be maintained in secrecy until the patents are issued. As a result, there may be prior art of which we or our licensors are not aware that may affect the validity or enforceability of a patent claim, and we or our licensors may be subject to priority disputes. For our in-licensed patent portfolios, we rely on our licensors to determine inventorship, and obtain and file inventor assignments of priority applications before their conversion as PCT applications. A failure to do so in a timely fashion may give rise to a challenge to entitlement of priority for foreign applications nationalized from such PCT applications. We or our licensors may in the future become a party to proceedings or priority disputes in Europe or other foreign jurisdictions. The loss of priority for, or the loss of, any European or other foreign patent rights could have a material adverse effect on the conduct of our business.
We may be required to disclaim part or all of the term of certain patent applications. There may be prior art of which we are not aware that may affect the validity or enforceability of a patent claim. There also may be prior art of which we or our licensors are aware, but which we or our licensors do not believe affects the validity or enforceability of a claim, which may, nonetheless, ultimately be found to affect the validity or enforceability of a claim. No assurance can be given that, if challenged, our patent applications, if issued, would be declared by a court, patent office or other governmental authority to be valid or enforceable or that even if found valid and enforceable, a competitor’s technology or product would be found by a court to infringe our patent rights. We may analyze patents or patent applications of our competitors that we believe are relevant to our activities, and consider that we are free to operate in relation to our product candidates, but our competitors may achieve issued claims, including in patents we consider to be unrelated, that block our efforts or potentially result in our product candidates or our activities infringing such claims. It is possible that our competitors may have filed, and may in the future file, patent applications covering our products or gene editing technology similar to ours. Those patent applications may have priority over our owned patent applications and in-licensed patent applications or patents, which could require us to obtain rights to issued patents covering such technologies. The possibility also exists that others will develop products that have the same effect as our product candidates on an independent basis that do not infringe our patents or other intellectual property rights, or will design around the claims of our patent applications or our in-licensed patents or patent applications that cover our product candidates.
Likewise, our currently owned patent applications, if issued as patents, and in-licensed patents and patent applications, if issued as patents, directed to our proprietary gene editing technologies and our product candidates are expected to expire from 2036 through 2043, without taking into account any possible patent term adjustments or extensions. Our owned or in-licensed patent applications, if issued as patents, may expire before, or soon after, our first product candidate achieves marketing approval in the United States or foreign jurisdictions. Additionally, no assurance can be given that the USPTO or relevant foreign patent offices will grant any of the pending patent applications we own or in-license currently or in the future. Upon the expiration of our current in-licensed patent applications, if issued as patents, we may lose the right to exclude others from practicing these inventions. The expiration of these patent rights could also have a similar material adverse effect on our business, financial condition, results of operations and prospects.
Our owned patent applications and in-licensed patents and patent applications and other intellectual property may be subject to inventorship or ownership disputes and similar proceedings. If we or our licensors are unsuccessful in any of these proceedings, we may be required to obtain licenses from third parties, which may not be available on commercially reasonable terms or at all, or
77
to cease the development, manufacture, and commercialization of one or more of our product candidates, which could have a material adverse impact on our business.
We or our licensors may also be subject to claims that former employees, collaborators, or other third parties have an interest in our owned or in-licensed patent applications or other intellectual property as an inventor or co-inventor. If we are unable to obtain an exclusive license to any such third-party co-owners’ interest in such patent applications, such co-owners may be able to license their rights to other third parties, including our competitors. In addition, we may need the cooperation of any such co-owners to enforce any patents that issue from such patent applications against third parties, and such cooperation may not be provided to us.
If we or our licensors are unsuccessful in any inventorship or ownership disputes to which we or they are subject, we may lose valuable intellectual property rights through the loss of part or all of our owned, licensed, or optioned patent rights, or such patent claims may be narrowed, invalidated, or held unenforceable, or through loss of exclusive ownership of or the exclusive right to use our owned or in-licensed patent rights. In the event of loss of patent rights as a result of any of these disputes, we may be required to obtain and maintain licenses from third parties, including parties involved in any such inventorship or ownership disputes. Such licenses may not be available on commercially reasonable terms or at all, or may be non-exclusive. If we are unable to obtain and maintain such licenses, we may need to cease the development, manufacture, and commercialization of one or more of our product candidates. The loss of exclusivity or the narrowing of our patent rights could limit our ability to stop others from using or commercializing similar or identical technology and product candidates. Even if we or our licensors are successful in an inventorship or ownership dispute, it could result in substantial costs and be a distraction to management and other employees. Any of the foregoing could result in a material adverse effect on our business, financial condition, results of operations, or prospects.
We have limited foreign intellectual property rights and may not be able to protect our intellectual property and proprietary rights throughout the world.
We may have limited intellectual property rights outside the United States. Filing, prosecuting, and defending patents on product candidates in all countries throughout the world would be prohibitively expensive, and our intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of foreign countries do not protect intellectual property rights to the same extent as federal and state laws of the United States. In addition, our intellectual property license agreements may not always include worldwide rights. Consequently, we may not be able to prevent third parties from practicing our inventions in all countries outside the United States, or from selling or importing products made using our inventions in and into the United States or other jurisdictions. Competitors may use our technologies in jurisdictions where we have not obtained patent protection to develop their own products and, further, may export otherwise infringing products to territories where we have patent protection but where enforcement is not as strong as that in the United States. These products may compete with our product candidates and our patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets, and other intellectual property protection, particularly those relating to biotechnology and pharmaceutical products, which could make it difficult for us to stop the infringement of our patents or marketing of competing products against third parties in violation of our intellectual property and proprietary rights generally. Proceedings to enforce our patents and intellectual property rights in foreign jurisdictions could result in substantial costs and divert our efforts and attention from other aspects of our business, could put our patents at risk of being invalidated or interpreted narrowly and our patent applications at risk of not issuing, and could provoke third parties to assert claims against us. We may not prevail in any lawsuits that we initiate, and the damages or other remedies awarded, if any, may not be commercially meaningful. Moreover, the initiation of proceedings by third parties to challenge the scope or validity of our patent rights in foreign jurisdictions could result in substantial cost and divert our efforts and attention from other aspects of our business. Accordingly, our efforts to enforce our intellectual property and proprietary rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that we develop or license.
Many countries have compulsory licensing laws under which a patent owner may be compelled to grant licenses to third parties. In addition, many countries limit the enforceability of patents against government agencies or government contractors. In these countries, the patent owner may have limited remedies, which could materially diminish the value of such patent. If we or our licensors are forced to grant one or more licenses to third parties with respect to any patents relevant to our business, our competitive position may be impaired, and our business, financial condition, results of operations, and prospects may be adversely affected.
If we fail to comply with our obligations in the agreements under which we license intellectual property rights from our licensors or otherwise experience disruptions to our business relationships with our licensors, we could lose license rights that are important to our business.
We have entered into a license agreement with Stanford related to our platform technology and certain of our product candidates, and a license agreement with Integrated DNA Technologies, Inc. (IDT) related to high- fidelity nucleases and gene editing systems, and may need to obtain licenses to additional intellectual property rights from these entities and others to advance our ongoing and planned research and development programs or to allow commercialization of our product candidates. It is possible that we may be unable to obtain any licenses to such additional intellectual property rights at a reasonable cost or on reasonable terms, if at all. In either event, we
78
may be required to expend significant time and resources to redesign our technology, product candidates, or the methods for manufacturing them or to develop or license replacement technology, all of which may not be feasible on a technical or commercial basis. If we are unable to do so, we may be unable to develop or commercialize the affected product candidates or expand our platform capabilities, which could harm our business, financial condition, results of operations, and prospects significantly. We cannot provide any assurances that third-party patents do not exist which might be enforced against our current technology, including gene editing technology, manufacturing methods, product candidates, or future methods or products resulting in either an injunction prohibiting our manufacture or future sales, or, with respect to our future sales, an obligation on our part to pay royalties and/or other forms of compensation to third parties, which could be significant.
Pursuant to our license agreements with Stanford and IDT, we are generally responsible for bringing any actions against any third party for infringing on the patent rights we have licensed. Certain provisions of our license agreements also require us to meet development thresholds to maintain the license, including establishing a set timeline for developing and commercializing products. In spite of our efforts, Stanford, IDT or any future licensor from whom we may seek to license intellectual property rights might conclude that we have materially breached our obligations under such license agreements and might therefore terminate the license agreements, thereby removing or limiting our ability to develop and commercialize products and technology covered by these license agreements. If these in-licenses are terminated, or if the underlying patent rights fail to provide the intended exclusivity, competitors or other third parties would have the freedom to seek regulatory approval of, and to market, products identical to ours and we may be required to cease our development and commercialization of our gene editing platform technology or product candidates. Any of the foregoing could have a material adverse effect on our competitive position, business, financial condition, results of operations, and growth prospects. Disputes may arise regarding intellectual property subject to a licensing agreement, including:
In addition, the agreements under which we currently license intellectual property rights from Stanford and IDT are complex, and certain provisions in such agreements may be susceptible to multiple interpretations. The resolution of any contract interpretation disagreement that may arise under our existing license agreements or future license agreements into which we may enter could narrow what we believe to be the scope of our rights to the relevant intellectual property or technology or broaden what we believe to be the scope of the licensor’s rights to our intellectual property and technology, or increase what we believe to be our financial or other obligations under the relevant agreement, any of which could have a material adverse effect on our business, financial condition, results of operations, and prospects. Moreover, if disputes over intellectual property that we have licensed prevent or impair our ability to maintain our current licensing arrangements on commercially acceptable terms, we may be unable to successfully develop and commercialize the affected product candidates. As a result, any termination of or disputes over our intellectual property license could result in the loss of our ability to develop and commercialize our gene editing platform or other product candidates or we could lose other significant rights, any of which could have a material adverse effect on our business, financial conditions, results of operations, and prospects. It is also possible that a third party could be granted limited licenses to some of the same technology, in certain circumstances.
We may not be successful in acquiring or in-licensing necessary rights to key technologies or any product candidates we may develop.
We currently have rights to intellectual property, through licenses from third parties, to identify and develop product candidates, and we expect to seek to expand our product candidate pipeline in part by in-licensing additional rights to key technologies. The future growth of our business will depend in part on our ability to in-license or otherwise acquire the rights to additional product candidates and technologies. Although we have succeeded in licensing technologies from Stanford and IDT as third party licensors in the past, we cannot assure you that we will be able to in-license or acquire additional rights to any product candidates or technologies from Stanford or other third parties on acceptable terms or at all. For example, there are third parties who possess technologies related to gene editing or other technologies which we may need to in-license.
In addition, our agreement with Stanford provides that our field of use is solely for the development of prophylactics and therapeutics for sickle cell disease, XSCID, and beta-thalassemia. If we determine that rights outside such field are necessary to commercialize our drug candidates or maintain our competitive advantage, we may need to obtain an additional license from Stanford
79
University in order to continue developing, manufacturing or marketing our drug candidates. We may not be able to obtain such a license on an exclusive basis, on commercially reasonable terms, or at all, which could prevent us from commercializing our drug candidates or allow our competitors or others the chance to access technology that is important to our business.
Furthermore, there has been extensive patenting activity in the field of gene editing, and pharmaceutical companies, biotechnology companies, and academic institutions are competing with us or are expected to compete with us in the in the field of gene editing technology and filing patent applications potentially relevant to our business and we are aware of certain third-party patent applications that, if issued, may allow the third party to circumvent our patent rights. For example, we are aware of several third-party patents, and patent applications, that if issued, may be construed to be relevant to our gene editing technology and product candidates. In order to market our product candidates, we may find it necessary or prudent to obtain licenses from such third-party intellectual property holders. However, we may be unable to secure such licenses or otherwise acquire or in-license any compositions, methods of use, processes, or other intellectual property rights from third parties that we identify as necessary for product candidates we may develop and gene editing technology. We may also require licenses from third parties for certain additional technologies, including technologies relating to gene editing such as guide RNA modification and target sequences, as well as technologies for cell manufacturing that we are evaluating for use with our product candidates. In addition, some of our in-licensed patent applications are co-owned with third parties. With respect to any patents co-owned with third parties, we may require licenses to such co-owners’ interest to such patents. If we are unable to obtain an exclusive license to any such third-party co-owners’ interest in such patent applications, such co-owners may be able to license their rights to other third parties, including our competitors, and our competitors could market competing products and technology. In addition, we may need the cooperation of any such co-owners of our patent applications in order to enforce such patent rights against third parties, and such cooperation may not be provided to us.
Additionally, we may collaborate with academic institutions to accelerate our preclinical research or development under written agreements with these institutions. In certain cases, these institutions provide us with an option to negotiate a license to any of the institution’s rights in technology resulting from the collaboration. Even if we hold such an option, we may be unable to negotiate a license from the institution within the specified timeframe or under terms that are acceptable to us. If we are unable to do so, the institution may offer the intellectual property rights to others, potentially blocking our ability to pursue our program.
For example, the licensing or acquisition of third-party intellectual property rights is a highly competitive area, and a number of more established companies are also pursuing strategies to license or acquire third-party intellectual property rights that we may consider attractive or necessary. These established companies may have a competitive advantage over us due to their size, capital resources and greater clinical development and commercialization capabilities. In addition, companies that perceive us to be a competitor may be unwilling to assign or license rights to us. We also may be unable to license or acquire third-party intellectual property rights on terms that would allow us to make an appropriate return on our investment or at all. If we are unable to successfully obtain rights to required third party intellectual property rights or maintain the existing intellectual property rights we have, we may have to abandon development of the relevant program or product candidate, which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
The intellectual property landscape around the technologies we use or plan to use, including gene editing technology, is highly dynamic, and third parties may initiate legal proceedings alleging that we are infringing, misappropriating, or otherwise violating their intellectual property rights, the outcome of which would be uncertain and may prevent, delay or otherwise interfere with our product discovery and development efforts.
Because of the large number of patents issued and patent applications filed in our field, third parties may allege they have patent rights encompassing our product candidates, technologies or methods. Third parties may assert that we are employing their proprietary technology without authorization and may file patent infringement claims or lawsuits against us, and if we are found to infringe such third-party patents, we may be required to pay damages, cease commercialization of the infringing technology, or obtain a license from such third parties, which may not be available on commercially reasonable terms or at all. In addition, we have in the past, and may in the future, receive an offer for license from third parties regarding their proprietary intellectual property for which they may believe encompass our product candidates and technologies. We will evaluate such offers for relevance to our business.
The field of gene editing is still in its infancy, and no such therapeutic product candidates have reached the market. Due to the intense research and development that is taking place by several companies, including us and our competitors, in this field, the intellectual property landscape is evolving and in flux, and it may remain uncertain for the coming years. There may be significant intellectual property related litigation and proceedings relating to our owned and in-licensed, and other third-party, intellectual property and proprietary rights in the future.
Our commercial success depends upon our ability and the ability of our collaborators and present and future licensors to develop, manufacture, market, and sell any product candidates that we may develop and use our proprietary technologies without infringing, misappropriating, or otherwise violating the intellectual property and proprietary rights of third parties. The biotechnology and pharmaceutical industries are characterized by extensive litigation regarding patents and other intellectual property rights as well as administrative proceedings for challenging patents, including interference, derivation, inter partes review, post grant review, and reexamination proceedings before the USPTO or oppositions and other comparable proceedings in foreign jurisdictions. We may be
80
subject to and may in the future become party to, or threatened with, adversarial proceedings or litigation regarding intellectual property rights with respect to our platform technology and our product candidates, including interference proceedings, post-grant review, inter partes review, and derivation proceedings before the USPTO and similar proceedings in foreign jurisdictions such as oppositions before the EPO. Numerous U.S. and foreign issued patents and pending patent applications that are owned by third parties exist in the fields in which we are developing our product candidates and they may assert infringement claims against us based on existing patents or patents that may be granted in the future, regardless of their merit.
As the biotechnology and pharmaceutical industries expand and more patents are issued, the risk increases that our platform technology and product candidates may give rise to claims of infringement of the patent rights of others. Moreover, it is not always clear to industry participants, including us, which patents cover various types of therapies, products or their methods of use or manufacture. There may also be third-party patents of which we are currently unaware with claims to technologies, methods of manufacture or methods for treatment related to the use or manufacture of our product candidates. Because patent applications can take many years to issue, there may be currently pending patent applications that may later result in issued patents that our product candidates may infringe. In addition, third parties may obtain patents in the future and claim that use of our technologies infringes upon these patents.
Numerous third-party U.S. and foreign issued patents and pending patent applications exist in the fields in which we are developing product candidates. Some of our product candidates make use of CRISPR-based technology, which is a field that is highly active for patent filings. As of June 2019, it was reported that approximately 2072 patent families worldwide related to CRISPR gene editing inventions and uses as the description and/or claims of these patent families specifically focus on a CRISPR-type system. The extensive patent filings related to CRISPR make it difficult for us to assess the full extent of relevant patents and pending applications that may cover our gene editing platform technology and product candidates and their use or manufacture. There may be third-party patents or patent applications with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacture of our gene editing platform technology and product candidates. For example, we are aware of a patent portfolio that is co-owned by the University of California, University of Vienna and Emmanuelle Charpentier, or the University of California Portfolio, which contains multiple patents and pending applications directed to gene editing. We are also aware of patents and patent applications directed to gene editing owned or co-owned by the Broad Institute, MIT and Harvard University, Toolgen, and Sigma Aldrich. Our ability to commercialize our product candidates may be adversely affected if we do not obtain a license to these patents. We may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be nonexclusive, thereby giving our competitors and other third parties access to the same technologies licensed to us, and it could require us to make substantial licensing and royalty payments. If we are unable to obtain a necessary license to a third-party patent on commercially reasonable terms, we may be unable to commercialize our gene editing platform technology or product candidates or such commercialization efforts may be significantly delayed, which could in turn significantly harm our business.
Our ability to commercialize our product candidates in the United States and abroad may be adversely affected if we cannot obtain a license on commercially reasonable terms to relevant third-party patents that cover our product candidates or platform technology. Even if we believe third-party intellectual property claims are without merit, there is no assurance that a court would find in our favor on questions of infringement, validity, enforceability, or priority. A court of competent jurisdiction could hold that these third-party patents are valid, enforceable, and infringed, which could materially and adversely affect our ability to commercialize our product candidates and any other product candidates or technologies covered by the asserted third-party patents. In order to successfully challenge the validity of any such U.S. patent in federal court, we would need to overcome a presumption of validity. As this burden is a high one requiring us to present clear and convincing evidence as to the invalidity of any such U.S. patent claim, there is no assurance that a court of competent jurisdiction would invalidate the claims of any such U.S. patent. If we are found to infringe a third-party’s intellectual property rights, and we are unsuccessful in demonstrating that such patents are invalid or unenforceable, we could be required to obtain a license from such third-party to continue developing, manufacturing, and marketing our product candidates and our technology. However, we may not be able to obtain any required license on commercially reasonable terms or at all. Even if we were able to obtain a license, it could be non-exclusive, thereby giving our competitors and other third parties access to the same technologies licensed to us, and it could require us to make substantial licensing and royalty payments. If we are unable to obtain a necessary license to a third-party patent on commercially reasonable terms, we may be unable to commercialize our platform technology or product candidates or such commercialization efforts may be significantly delayed, which could in turn significantly harm our business. We also could be forced, including by court order, to cease developing, manufacturing, and commercializing the infringing technology or product candidates. In addition, we could be found liable for significant monetary damages, including treble damages and attorneys’ fees, if we are found to have willfully infringed a patent or other intellectual property right. Claims that we have misappropriated the confidential information or trade secrets of third parties could have a similar material adverse effect on our business, financial condition, results of operations, and prospects.
Defense of third-party claims of infringement of misappropriation, or violation of intellectual property rights involves substantial litigation expense and would be a substantial diversion of management and employee time and resources from our business. Some third parties may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. In addition, any uncertainties resulting from the initiation and continuation of any litigation could have a material adverse effect on our ability to raise the funds necessary to continue our operations or could otherwise have a material adverse effect on our business, financial condition, results of operations and prospects. There could also be public announcements of the results of hearings,
81
motions, or other interim proceedings or developments, and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. Any of the foregoing events could have a material adverse effect on our business, financial condition, results of operations and prospects.
Third parties may assert that our employees or consultants have wrongfully used or disclosed confidential information or misappropriated trade secrets.
As is common in the biotechnology and biopharmaceutical industries, we employ individuals who were previously employed at universities or other biotechnology or biopharmaceutical companies, including our competitors or potential competitors. Although no claims against us are currently pending, and although we try to ensure that our employees and consultants do not use the proprietary information or know-how of others in their work for us, we may be subject to claims that we or our employees, consultants or independent contractors have inadvertently or otherwise used or disclosed intellectual property, including trade secrets or other proprietary information, of a former employer or other third parties. Litigation may be necessary to defend against these claims. If we fail in defending any such claims, in addition to paying monetary damages, we may lose valuable intellectual property rights or personnel. Even if we are successful in defending against such claims, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses, and could distract our technical and management personnel from their normal responsibilities. In addition, there could be public announcements of the results of hearings, motions or other interim proceedings or developments, and, if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. This type of litigation or proceeding could substantially increase our operating losses and reduce our resources available for development activities. We may not have sufficient financial or other resources to adequately conduct such litigation or proceedings. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their substantially greater financial resources. Uncertainties resulting from the initiation and continuation of patent litigation or other intellectual property related proceedings could adversely affect our ability to compete in the marketplace.
We may become involved in lawsuits to protect or enforce our future patents or the patents of our licensors, which could be expensive, time consuming, and unsuccessful and could result in a finding that such patents are unenforceable or invalid.
Competitors may infringe our future patents or the patents of our licensors, or we may be required to defend against claims of infringement. In addition, our future patents or the patents of our licensors also are, and may in the future become, involved in inventorship, priority, validity or enforceability disputes. Countering or defending against such claims can be expensive and time consuming. In an infringement proceeding, a court may decide that a patent owned or in-licensed by us is invalid or unenforceable, or may refuse to stop the other party from using the technology at issue on the grounds that our owned and in-licensed patents do not cover the technology in question. An adverse result in any litigation proceeding could put one or more of our owned or in-licensed patents at risk of being invalidated or interpreted narrowly.
In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace, and there are numerous grounds upon which a third party can assert invalidity or unenforceability of a patent. Third parties may also raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. These types of mechanisms include re-examination, post-grant review, inter partes review, interference proceedings, derivation proceedings, and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings). These types of proceedings could result in revocation or amendment to our patents such that they no longer cover our product candidates. The outcome for any particular patent following legal assertions of invalidity and unenforceability is unpredictable. With respect to the validity question, for example, we cannot be certain that there is no invalidating prior art, of which we, our licensors, our patent counsel and the patent examiner were unaware during prosecution. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, or if we are otherwise unable to adequately protect our rights, we would lose at least part, and perhaps all, of the patent protection on our technology and/or product candidates. Defense of these types of claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from our business.
Conversely, we may choose to challenge the patentability of claims in a third party’s U.S. patent by requesting that the USPTO review the patent claims in re-examination, post-grant review, inter partes review, interference proceedings, derivation proceedings, and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings). We may choose to challenge, third-party patents in patent opposition proceedings in the EPO or another foreign patent office. Even if successful, the costs of these opposition proceedings could be substantial, and may consume our time or other resources. If we fail to obtain a favorable result at the USPTO, EPO or other patent office then we may be exposed to litigation by a third party alleging that the patent may be infringed by our product candidates, platform technology or other proprietary technologies.
Even if resolved in our favor, litigation or other legal proceedings relating to intellectual property claims may cause us to incur significant expenses and could distract our personnel from their normal responsibilities. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential information could be compromised by disclosure during this type of litigation. In addition, there could be public announcements of the results of hearings, motions, or other interim proceedings or developments, and if securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock. Such litigation or proceedings could substantially increase
82
our operating losses and reduce the resources available for development activities or any future sales, marketing, or distribution activities. We may not have sufficient financial or other resources to conduct such litigation or proceedings adequately. Some of our competitors may be able to sustain the costs of such litigation or proceedings more effectively than we can because of their greater financial resources and more mature and developed intellectual property portfolios. Uncertainties resulting from the initiation and continuation of patent litigation or other proceedings could have a material adverse effect on our ability to compete in the marketplace.
Obtaining and maintaining our patent protection depends on compliance with various procedural, document submission, fee payment, and other requirements imposed by government patent agencies, and our patent protection could be reduced or eliminated for non-compliance with these requirements.
Periodic maintenance fees, renewal fees, annuity fees, and various other government fees on patents and applications are due to be paid to the USPTO and foreign patent agencies outside of the United States over the lifetime of our owned or licensed patents and applications. In certain circumstances, we rely on our licensors to pay these fees due to U.S. and non-U.S. patent agencies. The USPTO and foreign patent agencies require compliance with several procedural, documentary, fee payment, and other similar provisions during the patent application process. We are also dependent on our licensors to take the necessary action to comply with these requirements with respect to our licensed intellectual property. While an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with the applicable rules, there are situations, however, in which non-compliance can result a partial or complete loss of patent rights in the relevant jurisdiction. Were a noncompliance event to occur, our competitors might be able to enter the market with similar or identical products or technology, which could have a material adverse effect on our business, financial condition, results of operations, and prospects.
Changes in patent law in the United States and in non-U.S. jurisdictions could diminish the value of patents in general, thereby impairing our ability to protect our platform technology and product candidates.
As is the case with other biotech and pharmaceutical companies, our success is heavily dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involve both technological and legal complexity, and is therefore costly, time-consuming and inherently uncertain.
Changes in either the patent laws or interpretation of the patent laws could increase the uncertainties and costs surrounding the prosecution of patent applications and the enforcement or defense of our issued patents. For example, in March 2013, under the Leahy-Smith America Invents Act (America Invents Act), the United States transitioned from a “first to invent” to a “first-to-file” patent system. Under a “first-to-file” system, assuming that other requirements for patentability are met, the first inventor to file a patent application generally will be entitled to a patent on an invention regardless of whether another inventor had made the invention earlier. A third party that files a patent application in the USPTO after March 2013, but before us could therefore be awarded a patent covering an invention of ours even if we had made the invention before it was made by such third party. This will require us to be cognizant going forward of the time from invention to filing of a patent application. Since patent applications in the United States and most other countries are confidential for a period of time after filing or until issuance, we cannot be certain that we or our licensors were the first to either file any patent application related to our technology or product candidates or invent any of the inventions claimed in our or our licensors’ patents or patent applications. The America Invents Act also includes a number of other significant changes to U.S. patent law, including provisions that affect the way patent applications will be prosecuted, allowing third party submission of prior art and establish a new post-grant review system including post-grant review, inter partes review, and derivation proceedings. Because of a lower evidentiary standard in USPTO proceedings compared to the evidentiary standard in United States federal courts necessary to invalidate a patent claim, a third party could potentially provide evidence in a USPTO proceeding sufficient for the USPTO to hold a claim invalid even though the same evidence would be insufficient to invalidate the claim if first presented in a district court action. Accordingly, a third party may attempt to use the USPTO procedures to invalidate our patent claims that would not have been invalidated if first challenged by the third party as a defendant in a district court action. The effects of these changes are currently unclear as the USPTO continues to promulgate new regulations and procedures in connection with the America Invents Act and many of the substantive changes to patent law, including the “first-to-file” provisions, only became effective in March 2013. In addition, the courts have yet to address many of these provisions and the applicability of the act and new regulations on the specific patents discussed in this filing have not been determined and would need to be reviewed. However, the America Invents Act and its implementation could increase the uncertainties and costs surrounding the prosecution of our patent applications and the enforcement or defense of our issued patents.
In addition, recent U.S. Supreme Court rulings have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to our ability to obtain patents in the future, this combination of events has created uncertainty with respect to the validity and enforceability of patents, once obtained. Depending on future actions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that could weaken our ability to obtain new patents or to enforce our existing patents and patents that we might obtain in the future. For example, in the case, Assoc. for Molecular Pathology v. Myriad Genetics, Inc., the U.S. Supreme Court held that certain claims to DNA molecules are not patentable. We cannot predict how this and future decisions by the courts, the U.S. Congress or the USPTO may impact the value of our patents. Any similar adverse changes in the patent laws of other jurisdictions could also have a material adverse effect on our business, financial condition, results of operations and prospects.
83
Patent terms may be inadequate to protect our competitive position on our product candidates for an adequate amount of time.
Patents have a limited lifespan. The terms of individual patents depend upon the legal term for patents in the countries in which they are granted. In most countries, including the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest non-provisional filing date in the applicable country. However, the actual protection afforded by a patent varies from country to country, and depends upon many factors, including the type of patent, the scope of its coverage, the availability of regulatory- related extensions, the availability of legal remedies in a particular country and the validity and enforceability of the patent. Various extensions including PTE and PTA, may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering our product candidates are obtained, once the patent life has expired, we may be open to competition from competitive products, including generics or biosimilars. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting our product candidates might expire before or shortly after we or our partners commercialize those candidates. As a result, our owned and licensed patent portfolio may not provide us with sufficient rights to exclude others from commercializing products similar or identical to ours.
If we do not obtain PTE and data exclusivity for our product candidates, our business may be materially harmed.
Depending upon the timing, duration and specifics of any FDA marketing approval of our product candidates, one or more of our U.S. patents may be eligible for limited PTE under the Drug Price Competition and Patent Term Restoration Act of 1984 (Hatch-Waxman Amendments). The Hatch-Waxman Amendments PTE term of up to five years as compensation for patent term lost during the FDA regulatory review process. A PTE cannot extend the remaining term of a patent beyond a total of 14 years from the date of product approval, only one patent per product may be extended and only those claims covering the approved drug, a method for using it, or a method for manufacturing it may be extended. However, even if we were to seek a PTE, it may not be granted because of, for example, the failure to exercise due diligence during the testing phase or regulatory review process, the failure to apply within applicable deadlines, the failure to apply prior to expiration of relevant patents, or any other failure to satisfy applicable requirements. Moreover, the applicable time period or the scope of patent protection afforded could be less than we request. If we are unable to obtain PTE or term of any such extension is less than we request, our competitors may obtain approval of competing products following our patent expiration, and our business, financial condition, results of operations, and prospects could be materially harmed.
If we are unable to protect the confidentiality of our trade secrets, our business and competitive position would be harmed.
In addition to seeking patents for our technology and product candidates, we also rely on know-how and trade secret protection, as well as confidentiality agreements, non-disclosure agreements and invention assignment agreements with our employees, consultants and third-parties, to protect our confidential and proprietary information, especially where we do not believe patent protection is appropriate or obtainable.
It is our policy to require our employees, corporate collaborators, outside scientific collaborators, CROs, contract manufacturers, consultants, advisors, and other third parties to execute confidentiality agreements upon the commencement of employment or consulting relationships with us. These agreements provide that all confidential information concerning our business or financial affairs developed by or made known to the individual or entity during the course of the party’s relationship with us is to be kept confidential and not disclosed to third parties, except in certain specified circumstances. In the case of employees, the agreements provide that all inventions conceived by the individual, and that are related to our current or planned business or research and development or made during normal working hours, on our premises or using our equipment or proprietary information, are our exclusive property. In the case of consultants and other third parties, the agreements provide that all inventions conceived in connection with the services provided are our exclusive property. However, we cannot guarantee that we have entered into such agreements with each party that may have or have had access to our trade secrets or proprietary technology and processes. Additionally, the assignment of intellectual property rights may not be self-executing, or the assignment agreements may be breached, and we may be forced to bring claims against third parties, or defend claims that they may bring against us, to determine the ownership of what we regard as our intellectual property. Any of these parties may breach the agreements and disclose our proprietary information, including our trade secrets, and we may not be able to obtain adequate remedies for such breaches. Enforcing a claim that a party illegally disclosed or misappropriated a trade secret is difficult, expensive, and time-consuming, and the outcome is unpredictable.
In addition to contractual measures, we try to protect the confidential nature of our proprietary information through other appropriate precautions, such as physical and technological security measures. However, trade secrets and know-how can be difficult to protect. These measures may not, for example, in the case of misappropriation of a trade secret by an employee or third party with authorized access, provide adequate protection for our proprietary information. Our security measures may not prevent an employee or consultant from misappropriating our trade secrets and providing them to a competitor, and any recourse we might take against this type of misconduct may not provide an adequate remedy to protect our interests fully. In addition, trade secrets may be independently developed by others in a manner that could prevent us from receiving legal recourse. If any of our confidential or proprietary information, such as our trade secrets, were to be disclosed or misappropriated, or if any of that information was independently developed by a competitor, our competitive position could be harmed.
In addition, some courts inside and outside the United States are sometimes less willing or unwilling to protect trade secrets. If we choose to go to court to stop a third party from using any of our trade secrets, we may incur substantial costs. Even if we are
84
successful, these types of lawsuits may consume our time and other resources. Any of the foregoing could have a material adverse effect on our business, financial condition, results of operations and prospects.
If our trademarks and trade names are not adequately protected, then we may not be able to build name recognition in our markets of interest and our business may be adversely affected.
Our registered or unregistered trademarks or trade names may be challenged, infringed, circumvented or declared generic or determined to be infringing on other marks. We may not be able to protect our rights to these trademarks and trade names, which we need to build name recognition among potential partners or customers in our markets of interest. At times, competitors or other third parties may adopt trade names or trademarks similar to ours, thereby impeding our ability to build brand identity and possibly leading to market confusion. In addition, there could be potential trade name or trademark infringement claims brought by owners of other registered trademarks or trademarks that incorporate variations of our registered or unregistered trademarks or trade names. Over the long term, if we are unable to establish name recognition based on our trademarks and trade names, then we may not be able to compete effectively and our business may be adversely affected. Our efforts to enforce or protect our proprietary rights related to trademarks, trade secrets, domain names, copyrights or other intellectual property may be ineffective and could result in substantial costs and diversion of resources and could adversely affect our business, financial condition, results of operations and growth prospects.
Intellectual property rights do not necessarily address all potential threats.
The degree of future protection afforded by our intellectual property rights is uncertain because intellectual property rights have limitations and may not adequately protect our business or permit us to maintain our competitive advantage. For example:
85
Should any of these events occur, they could have a material adverse effect on our business, financial condition, results of operations, and prospects.
Risks Related to Regulatory and Other Legal Compliance Matters
Because gene editing is novel and the regulatory landscape that will govern our product candidates is uncertain and may change, we cannot predict the time and cost of obtaining regulatory approval, if we receive it at all, for our product candidates.
The regulatory requirements that will govern any novel gene editing product candidates we develop may continue to evolve. Within the broader genetic medicine field, a limited number of gene therapy products have received marketing authorization from the FDA and the EMA to date. Even with respect to more established products that fit into the categories of gene therapies or cell therapies, the regulatory landscape is still developing. Regulatory requirements governing gene therapy products and cell therapy products have changed frequently and may continue to change in the future. Moreover, there is substantial, and sometimes uncoordinated, overlap in those responsible for regulation of existing gene therapy products and cell therapy products. For example, in the United States in 2016, the FDA established the Office of Tissues and Advanced Therapies within its Center for Biologics Evaluation and Research (CBER), to consolidate the review of gene therapy and related products, and the Cellular, Tissue and Gene Therapies Advisory Committee to advise CBER on its review. In September 2022, the FDA announced retitling of OTAT to the Office of Therapeutic Products (OTP) and elevation of OTP to a “Super Office” to meet its growing cell and gene therapy workload. In addition, under guidelines issued by the National Institutes of Health (NIH) gene therapy clinical trials are also subject to review and oversight by an institutional biosafety committee (IBC), a local institutional committee that reviews and oversees basic and clinical research conducted at the institution participating in the clinical trial. While the NIH guidelines are not mandatory unless the research in question is being conducted at or sponsored by institutions receiving NIH funding of recombinant or synthetic nucleic acid molecule research, many companies and other institutions not otherwise subject to the NIH Guidelines voluntarily follow them. Although the FDA decides whether individual gene therapy protocols may proceed, the review process and determinations of other reviewing bodies, such as an IBC, can impede or delay the initiation of a clinical trial, even if the FDA has reviewed the trial and approved its initiation.
The same applies in the EU. The EMA’s Committee for Advanced Therapies (CAT), is responsible for assessing the quality, safety, and efficacy of advanced-therapy medicinal products (which include gene therapy medicines and somatic cell therapy medicines). The role of the CAT is to prepare a draft opinion on an application for marketing authorization for a gene therapy medicinal candidate that is submitted to the Committee for Medicinal Products for Human Use (CHMP), before the CHMP adopts its final opinion to send to the European Commission. In the European Union, the development and evaluation of a gene therapy medicinal products must be considered in the context of the relevant European Union guidelines. The EMA may issue new guidelines concerning the development and marketing authorization for gene therapy medicinal products and require that we comply with these new guidelines, which may increase our regulatory burden of developing and marketing gene and cell therapy products in the European Union.
Adverse developments in post-marketing experience or in clinical trials conducted by others of gene therapy products, cell therapy products, or products developed through the application of gene editing technology may cause the FDA, the EMA, and other regulatory bodies to revise the requirements for development or approval of our product candidates or limit the use of products utilizing gene editing technologies, either of which could materially harm our business. In addition, the clinical trial requirements of the FDA, the EMA, and other regulatory authorities and the criteria these regulators use to determine the safety and efficacy of a product candidate vary substantially according to the type, complexity, novelty, and intended use and market of the potential products. The regulatory approval process for novel product candidates such as our product candidates can be more expensive and take longer than for other, better known, or more extensively studied pharmaceutical or other product candidates. Regulatory agencies administering existing or
86
future regulations or legislation may not allow production and marketing of products utilizing gene editing technology in a timely manner or under technically or commercially feasible conditions. In addition, regulatory action or private litigation could result in expenses, delays, or other impediments to our research programs or the commercialization of resulting products.
The regulatory review committees and advisory groups described above and the new guidelines they promulgate may lengthen the regulatory review process, require us to perform additional studies or trials, increase our development costs, lead to changes in regulatory positions and interpretations, delay or prevent approval and commercialization of these treatment candidates, or lead to significant post-approval limitations or restrictions. As we advance our research programs and develop future product candidates, we will be required to consult with these regulatory and advisory groups and to comply with applicable guidelines. If we fail to do so, we may be required to delay or discontinue development of any product candidates we identify and develop.
Because we have been developing product candidates in the field of gene editing, in which there is limited clinical experience, there is increased risk that the FDA, the EMA, or other regulatory authorities may not consider the endpoints of our clinical trials to provide clinically meaningful results and that these results may be difficult to analyze.
During the regulatory review process, we will need to identify success criteria and endpoints such that the FDA, the EMA, or other regulatory authorities will be able to determine the clinical efficacy and safety profile of our product candidates. As we have been initially seeking to identify and develop product candidates to treat diseases in which there is little clinical experience using new technologies, there is heightened risk that the FDA, the EMA, or other regulatory authorities may not consider the clinical trial endpoints that we propose to provide clinically meaningful results (reflecting a tangible benefit to patients). In addition, the resulting clinical data and results may be difficult to analyze. Even if the FDA does find our success criteria to be sufficiently validated and clinically meaningful, we may not achieve the pre-specified endpoints to a degree of statistical significance. This may be a particularly significant risk for many of the genetically defined diseases for which we have developed or plan to develop product candidates because many of these diseases, including SCD, XSCID and Gaucher disease, have small patient populations, and designing and executing a rigorous clinical trial with appropriate statistical power is more difficult than with diseases that have larger patient populations. Further, even if we do achieve the pre-specified criteria, we may produce results that are unpredictable or inconsistent with the results of the non-primary endpoints or other relevant data. The FDA also weighs the benefits of a product against its risks, and the FDA may view the efficacy results in the context of safety as not being supportive of regulatory approval. Other regulatory authorities in the European Union and other countries may make similar comments with respect to these endpoints and data. Our product candidates will be based on a novel technology that makes it difficult to predict the time and cost of development and of subsequently obtaining regulatory approval. No gene editing therapeutic product has been approved in the United States or in Europe.
Clinical development involves a lengthy and expensive process, with an uncertain outcome. If clinical trials of any of our current or future product candidates fail to demonstrate safety and efficacy to the satisfaction of regulatory authorities or do not otherwise produce positive results, we may incur additional costs or experience delays in completing, or ultimately be unable to complete, the development and commercialization of such product candidates.
In February 2023, we discontinued our clinical development of nula-cel in SCD, and all of our other programs are in discovery or preclinical development. Before obtaining marketing approval from regulatory authorities for the sale of our product candidates, we must complete preclinical development and then conduct extensive clinical trials to demonstrate the safety and efficacy of the product in humans. It is impossible to predict when or if any of our programs will prove effective and safe in humans or will receive regulatory approval. Preclinical and clinical testing is expensive, difficult to design and implement, can take many years to complete and is uncertain as to outcome. A failure of one or more clinical trials can occur at any stage of clinical trials. Interim results of a clinical trial do not necessarily predict final results. We do not know whether any of our clinical trials will begin or be completed on schedule, if at all.
Moreover, preclinical and clinical data are often susceptible to varying interpretations and analyses. Many companies that have believed their product candidates performed satisfactorily in preclinical studies and clinical trials have nonetheless failed to obtain marketing approval of their product candidates.
We may experience numerous unforeseen events during, or as a result of, clinical trials that we conduct, which could delay or prevent our ability to receive marketing approval or commercialize our product candidates, including:
87
We could also encounter delays if a clinical trial is suspended or terminated by us, the IRBs of the institutions in which such trials are being conducted or the relevant ethics committee, the Data Safety Monitoring Board (DSMB), for such trial, or the FDA or other relevant regulatory authorities. We or such authorities may impose such a suspension or termination due to a number of factors, including failure to conduct the clinical trial in accordance with regulatory requirements or our clinical protocols, inspection of the clinical trial operations or trial site by the FDA or other regulatory authorities, resulting in the imposition of a clinical hold, manufacturing or quality control issues, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from using a product or treatment, failure to establish or achieve clinically meaningful trial endpoints, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. Many of the factors that cause, or lead to, a delay in the commencement or completion of clinical trials may also ultimately lead to the denial of regulatory approval of our product candidates. Further, the FDA or other regulatory authorities may disagree with our clinical trial design and our interpretation of data from clinical trials or may change the requirements for approval even after they have reviewed and commented on the design for our clinical trials.
If we are required to conduct additional clinical trials or other testing of our product candidates beyond those that we currently contemplate, if we are unable to successfully complete clinical trials or other testing of our product candidates, or if the results of these trials or tests are not positive or are only modestly positive or if there are safety concerns, we may:
88
Product development costs will also increase if we or our collaborators experience delays in clinical trials or other testing or in obtaining marketing approvals. We do not know whether any clinical trials will begin as planned, will need to be restructured, or will be completed on schedule, or at all. Significant clinical trial delays also could shorten any periods during which we may have the exclusive right to commercialize our product candidates, could allow our competitors to bring products to market before we do, and could impair our ability to successfully commercialize our product candidates, any of which may harm our business, financial condition, results of operations, and prospects.
Failure to obtain marketing approval in foreign jurisdictions would prevent our product candidates from being marketed in such jurisdictions, which, in turn, would materially impair our ability to generate revenue.
In order to market and sell our product candidates in the European Union and other foreign jurisdictions, we or our third-party collaborators must obtain separate marketing approvals (a single one for the European Union) and comply with numerous and varying regulatory requirements. The approval procedure varies among countries and can involve additional testing. The time required to obtain approval may differ substantially from that required to obtain FDA approval. The regulatory approval process outside the United States generally includes all of the risks associated with obtaining FDA approval. In addition, in many countries outside the United States, it is required that the product candidate be approved for reimbursement before the product candidate can be approved for sale in that country. We or these third parties may not obtain approvals from regulatory authorities outside the United States on a timely basis, if at all. Approval by the FDA does not ensure approval by regulatory authorities in other countries or jurisdictions, and approval by one regulatory authority outside the United States does not ensure approval by regulatory authorities in other countries or jurisdictions or by the FDA. We may not be able to file for marketing approvals and may not receive necessary approvals to commercialize our product candidates in any jurisdiction, which would materially impair our ability to generate revenue.
The withdrawal of the United Kingdom from the EU formally took effect on January 31, 2020 under the terms of the Withdrawal Agreement. The United Kingdom and the EU have signed a EU-UK Trade and Cooperation Agreement, which became provisionally applicable on January 1, 2021 and has been formally applicable since May 1, 2021. Under the terms of the deal, the EU and UK have separate regulatory regimes for pharmaceutical products, although there are some provisions for mutual recognition of standards, for example with regards to GMP. For instance, the UK is no longer covered by the centralized procedure for obtaining EU-wide marketing authorizations for medicinal products, and a separate process for authorization of medicinal products is required in the UK, resulting in an authorization covering the UK or Great Britain (England, Scotland and Wales) only. However, notwithstanding that there is no wholesale recognition of EU pharmaceutical legislation under the TCA, the MHRA has announced it is putting in place a new framework from January 1, 2024, whereby the MHRA will take into account decisions on the approval of marketing authorizations from the EMA (and certain other regulators) when considering an application for a Great Britain marketing authorization. At present, Great Britain has implemented EU legislation on the marketing, promotion and sale of medicinal products through the Human Medicines Regulations 2012 (as amended) (under the Northern Ireland Protocol, the EU regulatory framework will continue to apply in Northern Ireland for a period of time, until the new Windsor Framework comes into effect). The regulatory regime in Great Britain therefore broadly aligns with current EU regulations, however longer term, the United Kingdom is likely to develop its own legislation that diverges from that in the EU now that Great Britain’s regulatory system is independent from the EU and the TCA does not provide for mutual recognition of UK and EU pharmaceutical legislation. It is possible therefore, that there will be increased regulatory complexities in the UK and EU, which could disrupt the timing of any clinical trials and regulatory approvals that we may determine to pursue in these jurisdictions.
Even if we, or any collaborators we may have, obtain marketing approvals for our product candidates, the terms of approvals and ongoing regulation of our product candidates could require the substantial expenditure of resources and may limit how we, or they, manufacture and market our product candidates, which could materially impair our ability to generate revenue.
Any product candidate for which we obtain marketing approval, along with the manufacturing processes, post-approval clinical data, labeling, advertising, and promotional activities for such product, will be subject to continual requirements of and review by the FDA, EMA and other regulatory authorities. These requirements include submissions of safety and other post-marketing information and reports, facility registration and drug listing requirements, cGMP requirements relating to quality control, quality assurance and corresponding maintenance of records and documents, applicable product tracking and tracing requirements, and requirements regarding the distribution of samples to physicians and recordkeeping. Even if marketing approval of a product candidate is granted, the approval may be subject to limitations on the indicated uses for which the products may be marketed or to the conditions of approval, or contain requirements for costly post-marketing testing and surveillance to monitor the safety or efficacy of the products.
89
Accordingly, assuming we, or any collaborators we may have, receive marketing approval for one or more product candidates we develop, we, and such collaborators, and our and their contract manufacturers will continue to expend time, money, and effort in all areas of regulatory compliance, including manufacturing, production, product surveillance, and quality control. If we and such collaborators are not able to comply with post-approval regulatory requirements, we and such collaborators could have the marketing approvals for our products withdrawn by regulatory authorities and our, or such collaborators’, ability to market any future products could be limited, which could adversely affect our ability to achieve or sustain profitability. Further, the cost of compliance with post-approval regulations may have a negative effect on our business, operating results, financial condition, and prospects.
Any product candidate for which we obtain marketing approval could be subject to restrictions or withdrawal from the market, and we may be subject to substantial penalties if we fail to comply with regulatory requirements or if we experience unanticipated problems with our product candidates, when and if any of them are approved.
The FDA, the EMA, and other regulatory agencies closely regulate the post-approval marketing and promotion of product candidates to ensure that they are marketed only for the approved indications and in accordance with the provisions of the approved labeling. The FDA, the EMA and other regulatory agencies impose stringent restrictions on manufacturers’ communications regarding off-label use, and if we market our product candidates for off-label use, we may be subject to enforcement action for off-label marketing by the FDA and other federal and state enforcement agencies, including the Department of Justice. Violation of the Federal Food, Drug, and Cosmetic Act (FDCA), and other statutes, including the False Claims Act (FCA), and equivalent legislation in other countries relating to the promotion and advertising of prescription products may also lead to investigations or allegations of violations of federal and state and other countries’ health care fraud and abuse laws and state consumer protection laws. Even if it is later determined we were not in violation of these laws, we may be faced with negative publicity, incur significant expenses defending our actions and have to divert significant management resources from other matters.
In addition, later discovery of previously unknown problems with our products, manufacturers, or manufacturing processes, or failure to comply with regulatory requirements, may yield various negative consequences, including:
Any government investigation of alleged violations of law could require us to expend significant time and resources in response and could generate negative publicity. The occurrence of any event or penalty described above may inhibit our ability to commercialize our product candidates and adversely affect our business, financial condition, results of operations, and prospects.
Our relationships with healthcare providers, physicians, and third-party payors will be subject to applicable anti-kickback, fraud and abuse, anti-bribery and other healthcare laws and regulations, which could expose us to criminal sanctions, civil penalties, contractual damages, reputational harm, and diminished profits and future earnings.
Healthcare providers, including physicians, and third-party payors play a primary role in the recommendation and prescription of any product candidates that we may develop for which we obtain marketing approval. Our current and future arrangements with third-party payors, healthcare providers and customers may expose us to broadly applicable fraud and abuse and other healthcare laws and regulations that may constrain the business or financial arrangements and relationships through which we market, sell, and
90
distribute our products for which we obtain marketing approval. See section entitled “Business – Government Other U.S. Healthcare Laws and Compliance Requirements.”
Efforts to ensure that our business arrangements with third parties will comply with applicable healthcare laws and regulations will involve substantial costs. Given the breadth of the laws and regulations, limited guidance for certain laws and regulations and evolving government interpretations of the laws and regulations, governmental authorities may possibly conclude that our business practices may not comply with healthcare laws and regulations. If our operations are found to be in violation of any of the laws described above or any other government regulations that apply to us, we may be subject to significant penalties, including without limitation, civil, criminal and/or administrative penalties, damages, fines, disgorgement, exclusion from participation in government health care programs, such as Medicare and Medicaid, individual imprisonment, injunctions, private “qui tam” actions brought by individual whistleblowers in the name of the government, or refusal to allow us to enter into government contracts, contractual damages, reputational harm, administrative burdens, diminished profits and future earnings, and the curtailment or restructuring of our operations, any of which could adversely affect our business, financial condition, results of operations, and prospects.
Healthcare and other reform legislation may increase the difficulty and cost for us and any collaborators we may have to obtain marketing approval of and commercialize our product candidates and affect the prices we, or they, may obtain.
In the United States and some foreign jurisdictions, there have been and continue to be ongoing efforts to implement legislative and regulatory changes regarding the healthcare system. Such changes could prevent or delay marketing approval of any product candidates that we may develop, restrict or regulate post-approval activities, and affect our ability to profitably sell any product candidates for which we obtain marketing approval. Although we cannot predict what healthcare or other reform efforts will be successful, such efforts may result in more rigorous coverage criteria, in additional downward pressure on the price that we, or our future collaborators, may receive for any approved products or in other consequences that may adversely affect our ability to achieve or maintain profitability. See section entitled “Business – Government Regulation – Healthcare Reform.”
The continuing efforts of the government, insurance companies, managed care organizations and other payers of healthcare services to contain or reduce costs of healthcare may adversely affect:
Within the United States, the federal government and individual states have aggressively pursued healthcare reform, as evidenced by the passing of the ACA and the ongoing efforts to modify or repeal that legislation. The ACA substantially changed the way healthcare is financed by both governmental and private insurers and contains a number of provisions that affect coverage and reimbursement of drug products and/or that could potentially reduce the demand for pharmaceutical products such as increasing drug rebates under state Medicaid programs for brand name prescription drugs and extending those rebates to Medicaid managed care and assessing a fee on manufacturers and importers of brand name prescription drugs reimbursed under certain government programs, including Medicare and Medicaid. Other aspects of healthcare reform, such as expanded government enforcement authority and heightened standards that could increase compliance-related costs, could also affect our business. Modifications have been implemented under the previous presidential administration and additional modifications or repeal may occur.
Moreover, increasing efforts by governmental and third-party payors in the United States and abroad to cap or reduce healthcare costs may cause such organizations to limit both coverage and the level of reimbursement for newly approved products and, as a result, they may not cover or provide adequate payment for our product candidates. There has been increasing legislative and enforcement interest in the United States with respect to specialty drug pricing practices. Specifically, there have been several recent U.S. Congressional inquiries and proposed and enacted federal and state legislation designed to, among other things, bring more transparency to drug pricing, reduce the cost of prescription drugs under Medicare, review the relationship between pricing and manufacturer patient programs, and reform government program reimbursement methodologies for drugs.
91
We expect that the healthcare reform measures that have been adopted and may be adopted in the future, may result in more rigorous coverage criteria and in additional downward pressure on the price that we receive for any approved product and could seriously harm our future revenues. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. The implementation of cost containment measures or other healthcare reforms may prevent us from being able to generate revenue, attain profitability or commercialize our products.
Fast track, breakthrough, or regenerative medicine advanced therapy designation by the FDA may not actually lead to a faster development or regulatory review or approval process, and does not assure FDA approval of our product candidates.
FDA’s fast track, breakthrough, and regenerative medicine advanced therapy (RMAT), programs are intended to expedite the development of certain qualifying products intended for the treatment of serious diseases and conditions. If a product candidate is intended for the treatment of a serious or life-threatening condition and preclinical or clinical data demonstrate the product’s potential to address an unmet medical need for this condition, the sponsor may apply for FDA fast track designation. A product candidate may be designated as a breakthrough therapy if it is intended to treat a serious or life-threatening condition and preliminary clinical evidence indicates that the product candidate may demonstrate substantial improvement over existing therapies on one or more clinically significant endpoints. A product candidate may receive RMAT designation if it is a regenerative medicine therapy that is intended to treat, modify, reverse or cure a serious or life-threatening condition, and preliminary clinical evidence indicates that the product candidate has the potential to address an unmet medical need for such condition. While we may seek fast track, breakthrough, and/or RMAT designation, there is no guarantee that we will be successful in obtaining any such designation. Even if we do obtain such designation, we may not experience a faster development process, review or approval compared to conventional FDA procedures. A fast track, breakthrough, or RMAT designation does not ensure that the product candidate will receive marketing approval or that approval will be granted within any particular timeframe. In addition, the FDA may withdraw fast track, breakthrough, or RMAT designation if it believes that the designation is no longer supported by data from our clinical development program. Fast track, breakthrough, and/or RMAT designation alone do not guarantee qualification for the FDA’s priority review procedures.
Priority review designation by the FDA may not lead to a faster regulatory review or approval process and, in any event, does not assure FDA approval of our product candidates.
If the FDA determines that a product candidate is intended to treat a serious disease or condition and, if approved, would provide a significant improvement in the safety or effectiveness of the treatment, prevention, or diagnosis of such disease or condition, the FDA may designate the product candidate for priority review. A priority review designation means that the goal for the FDA to review a marketing application is six months from filing of the application, rather than the standard review period of ten months. We may request priority review for certain of our product candidates. The FDA has broad discretion with respect to whether or not to grant priority review status to a product candidate, so even if we believe a particular product candidate is eligible for such designation or status, the FDA may disagree and decide not to grant it. Moreover, a priority review designation does not necessarily mean a faster regulatory review process or necessarily confer any advantage with respect to approval compared to conventional FDA procedures. Receiving priority review from the FDA does not guarantee approval within the six-month review cycle or thereafter.
We may not be able to obtain orphan drug designation for our product candidates, and even if we do, we may not be able to obtain orphan drug exclusivity and the associated market exclusivity may not prevent the FDA or the EMA from approving other competing products.
Under the Orphan Drug Act, the FDA may designate a product candidate as an orphan drug if it is a drug or biologic intended to treat a rare disease or condition. A similar regulatory scheme governs approval of orphan product candidates by the EMA in the European Union. Generally, if a product with an orphan drug designation subsequently receives the first marketing approval for the indication for which it has such designation, the product is entitled to a period of marketing exclusivity, which precludes the FDA or the EMA from approving another marketing authorization application for another similar product candidate for the same orphan therapeutic indication for that time period. The applicable period is seven years in the United States and ten years in the European Union. The exclusivity period in the European Union can be reduced to six years if a product no longer meets the criteria for orphan drug designation, in particular if the product is sufficiently profitable so that market exclusivity is no longer justified.
In order for the FDA to grant orphan drug designation to one of our product candidates, the agency must find that the product candidate is intended to treat a condition or disease that affects fewer than 200,000 individuals in the United States or that affects 200,000 or more individuals in the United States and for which there is no reasonable expectation that the cost of developing and making the product candidate available for the disease or condition will be recovered from sales of the product in the United States. The FDA may conclude that the condition or disease for which we seek orphan drug designation does not meet this standard. Even if a product candidate we are developing is approved, we may not obtain orphan drug exclusivity. If we obtain orphan drug exclusivity for a product candidate, that exclusivity may not effectively protect the product candidate from competition because different product candidates can be approved for the same condition. In addition, even after an orphan drug is approved, the FDA can subsequently approve the same product candidate for the same condition if the FDA concludes that the later product candidate is clinically superior in that it is shown to be safer, more effective or makes a major contribution to patient care compared with the product that has orphan exclusivity. Orphan drug exclusivity
92
may also be lost if the FDA or EMA determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the product to meet the needs of the patients with the rare disease or condition.
Our employees, principal investigators, consultants, and commercial partners may engage in misconduct or other improper activities, including non-compliance with regulatory standards and requirements and insider trading.
We are exposed to the risk of fraud or other misconduct by our employees, consultants, and commercial partners, and, if we commence clinical trials, our principal investigators. Misconduct by these parties could include intentional failures to comply with FDA regulations or the regulations applicable in the European Union and other jurisdictions, provide accurate information to the FDA, the EMA, and other regulatory authorities, comply with healthcare fraud and abuse laws and regulations in the United States and abroad, report financial information or data accurately, or disclose unauthorized activities to us. In particular, sales, marketing, and business arrangements in the healthcare industry are subject to extensive laws and regulations intended to prevent fraud, misconduct, kickbacks, self-dealing and other abusive practices. These laws and regulations restrict or prohibit a wide range of pricing, discounting, marketing and promotion, sales commission, customer incentive programs, and other business arrangements. Such misconduct also could involve the improper use of information obtained in the course of clinical trials or interactions with the FDA, the EMA or other regulatory authorities, which could result in regulatory sanctions and cause serious harm to our reputation. We have adopted a code of conduct applicable to all of our employees, but it is not always possible to identify and deter employee misconduct, and the precautions we take to detect and prevent this activity may not be effective in controlling unknown or unmanaged risks or losses or in protecting us from government investigations or other actions or lawsuits stemming from a failure to comply with these laws or regulations. If any such actions are instituted against us, and we are not successful in defending ourselves or asserting our rights, those actions could have a significant impact on our business, financial condition, results of operations, and prospects, including the imposition of significant fines or other sanctions.
We are subject to U.S. and certain foreign export and import controls, sanctions, embargoes, anti-corruption laws, and anti-money laundering laws and regulations. Compliance with these legal standards could impair our ability to compete in domestic and international markets. We can face criminal liability and other serious consequences for violations, which can harm our business.
We are subject to export control and import laws and regulations, including the U.S. Export Administration Regulations, U.S. Customs regulations, various economic and trade sanctions regulations administered by the U.S. Treasury Department’s Office of Foreign Assets Control, the U.S. Foreign Corrupt Practices Act of 1977, as amended (FCPA), the U.S. domestic bribery statute contained in 18 U.S.C. § 201, the U.S. Travel Act, the USA PATRIOT Act, and other state and national anti-bribery and anti-money laundering laws in the countries in which we conduct activities. Anti-corruption laws are interpreted broadly and prohibit companies and their employees, agents, contractors, and other collaborators from authorizing, promising, offering, or providing, directly or indirectly, improper payments or anything else of value to recipients in the public or private sector. We may engage third parties to sell our products outside the United States, to conduct clinical trials, and/or to obtain necessary permits, licenses, patent registrations, and other regulatory approvals. We have direct or indirect interactions with officials and employees of government agencies or government-affiliated hospitals, universities, and other organizations. We can be held liable for the corrupt or other illegal activities of our employees, agents, contractors, and other collaborators, even if we do not explicitly authorize or have actual knowledge of such activities. Any violations of the laws and regulations described above may result in substantial civil and criminal fines and penalties, imprisonment, the loss of export or import privileges, debarment, tax reassessments, breach of contract and fraud litigation, reputational harm, and other consequences.
Inadequate funding for the FDA and other government agencies could hinder their ability to hire and retain key leadership and other personnel, prevent new products and services from being developed or commercialized in a timely manner or otherwise prevent those agencies from performing normal business functions on which the operation of our business may rely, which could negatively impact our business.
The ability of the FDA to review and approve new products can be affected by a variety of factors, including government budget and funding levels, ability to hire and retain key personnel and accept the payment of user fees, and statutory, regulatory, and policy changes. Average review times at the agency have fluctuated in recent years as a result. In addition, government funding of other agencies on which our operations may rely, including those that fund research and development activities, is subject to the political process, which is inherently fluid and unpredictable.
Disruptions at the FDA and other agencies may also slow the time necessary for new drugs to be reviewed and/or approved by necessary government agencies, which would adversely affect our business. For example, over the last several years the U.S. government has shut down several times and certain regulatory agencies, such as the FDA, have had to furlough critical FDA and other government employees and stop critical activities. Since March 2020 when foreign and domestic inspections of facilities were largely placed on hold, the FDA has been working to resume pre-pandemic levels of inspection activities, including routine surveillance, bioresearch monitoring and pre-approval inspections. Should FDA determine that an inspection is necessary for approval and an inspection cannot be completed during the review cycle due to restrictions on travel, and the FDA does not determine a remote interactive evaluation to be adequate, the agency has stated that it generally intends to issue, depending on the circumstances, a complete response letter or defer action on the application until an inspection can be completed. During the COVID-19 public health emergency, a number of companies
93
announced receipt of complete response letters due to the FDA’s inability to complete required inspections for their applications. Regulatory authorities outside the U.S. may adopt similar restrictions or other policy measures in response to the ongoing COVID-19 pandemic and may experience delays in their regulatory activities.
We are subject to stringent privacy laws, information security laws, regulations, policies and contractual obligations related to data privacy and security and changes in such laws, regulations, policies and contractual obligations could adversely affect our business.
We are subject to data privacy and protection laws and regulations that apply to the collection, transmission, storage and use of personally identifying information, which among other things, impose certain requirements relating to the privacy, security and transmission of personal information, including comprehensive regulatory systems in the United States. The legislative and regulatory landscape for privacy and data protection continues to evolve in jurisdictions worldwide, and there has been an increasing focus on privacy and data protection issues with the potential to affect our business. Failure to comply with any of these laws, regulations, contractual obligations, or standards could result in enforcement action against us, including fines, imprisonment of company officials and public censure, claims for damages by affected individuals, customers, or business partners, damage to our reputation and loss of goodwill, any of which could have a material adverse effect on our business, financial condition, results of operations or prospects.
There are numerous U.S. federal and state laws and regulations related to the privacy and security of personal information. In particular, regulations promulgated pursuant to the HIPAA establish privacy and security standards that limit the use and disclosure of individually identifiable health information, or protected health information, and require the implementation of administrative, physical and technological safeguards to protect the privacy of protected health information and ensure the confidentiality, integrity and availability of electronic protected health information. Determining whether protected health information has been handled in compliance with applicable privacy standards and our contractual obligations can be complex and may be subject to changing interpretation.
If we are unable to properly protect the privacy and security of protected health information, we could be alleged or actually found to have breached our contracts. Further, if we fail to comply with applicable privacy laws, including applicable HIPAA privacy and security standards, we could face civil and criminal penalties. The U.S. Department of Health and Human Services, of HHS, has the discretion to impose penalties without attempting to resolve violations through informal means. HHS enforcement activity can result in financial liability and reputational harm, and responses to such enforcement activity can consume significant internal resources. In addition, state attorneys general are authorized to bring civil actions seeking either injunctions or damages in response to violations that threaten the privacy of state residents. Furthermore, a number of states, including California, Virginia and Colorado have enacted comprehensive consumer privacy laws. Although these laws contain exemptions for information subject to HIPAA, we cannot be certain how these new laws will be interpreted, enforced or applied to our operations. In addition to the risks associated with enforcement activities and potential contractual liabilities, our ongoing efforts to comply with evolving laws and regulations at the federal and state level may be costly and require ongoing modifications to our policies, procedures and systems.
Data privacy remains an evolving landscape at both the domestic and international level, with new regulations coming into effect and continued legal challenges, and our efforts to comply with the evolving data protection rules may be unsuccessful. It is possible that these laws may be interpreted and applied in a manner that is inconsistent with our practices. We must devote significant resources to understanding and complying with this changing landscape. Failure to comply with laws regarding privacy and/or data protection would expose us to risk of enforcement actions taken by data protection authorities and carries with it the potential for significant penalties if we are found to be non-compliant. Similarly, failure to comply with federal and state laws in the United States regarding privacy and security of personal information could expose us to penalties under such laws. Any such failure to comply with data protection and privacy laws could result in government-imposed fines or orders requiring that we change our practices, claims for damages or other liabilities, regulatory investigations and enforcement action, litigation and significant costs for remediation, any of which could adversely affect our business. Even if we are not determined to have violated these laws, government investigations into these issues typically require the expenditure of significant resources and generate negative publicity, which could harm our business, financial condition, results of operations or prospects.
Risks Related to Employee Matters, Managing Growth, Public Health and Information Technology
Our future success depends on our ability to retain our executive officers and other key employees and to attract, retain, and motivate qualified personnel.
We are highly dependent on our executive officers, as well as the other principal members of our management and scientific teams. Each of our executive officers and such other principal members are employed “at will,” meaning we or they may terminate the employment at any time. We do not maintain “key person” insurance for any of our executives or other employees. The loss of the services of any of these persons could impede the achievement of our research, development, and commercialization objectives.
Recruiting and retaining qualified scientific, clinical, manufacturing, and sales and marketing personnel is critical to our success. We may not be able to attract and retain these personnel on acceptable terms given the competition among numerous pharmaceutical and biotechnology companies for similar personnel. We also experience competition for the hiring of scientific and clinical personnel
94
from universities and research institutions. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research and development and commercialization strategy. Our consultants and advisors, including our scientific co-founders, may be employed by employers other than us and may have commitments under consulting or advisory contracts with other entities that may limit their availability to us. The inability to recruit, or loss of services of certain executives, key employees, consultants, or advisors, may impede the progress of our research, development, and commercialization objectives and have a material adverse effect on our business, financial condition, results of operations, and prospects.
Our internal computer systems, or those of our third-party vendors, collaborators or other contractors or consultants, may fail or suffer security breaches, which could result in a material disruption of our product development programs, compromise sensitive information related to our business or prevent us from accessing critical information, potentially exposing us to liability or otherwise adversely affecting our business.
Our internal computer systems and those of our current and any future third-party vendors, collaborators and other contractors or consultants are vulnerable to damage or interruption from computer viruses, malware (including ransomware), phishing attacks, computer hackers, malicious code, employee theft or misuse, intentional or accidental action or lack of action by our employees or any contractors with access to our systems that leads to the introduction of vulnerabilities, denial-of-service attacks, sophisticated nation-state and nation- state-supported actors, supply chain attacks, unauthorized access, natural disasters, terrorism, war and telecommunication and electrical failures. While we seek to protect our information technology systems from system failure and we seek to identify and manage specific cyber security risks, accident and security breach, if such an event were to occur and cause interruptions in our operations, it could result in a disruption of our development programs and our business operations, whether due to a loss of our trade secrets, personal information, or other proprietary information or other disruptions. For example, the loss of clinical trial data from future clinical trials could result in delays in our regulatory approval efforts and significantly increase our costs to recover or reproduce the data. If we were to experience a significant cybersecurity breach of our information systems or data, the costs associated with the investigation, remediation and potential notification of the breach to counter-parties and data subjects could be material. In addition, our remediation efforts may not be successful. If we do not allocate and effectively manage the resources necessary to build and sustain the proper technology and cybersecurity infrastructure, we could suffer significant business disruption, including transaction errors, supply chain or manufacturing interruptions, processing inefficiencies, data loss or the loss of or damage to intellectual property or other proprietary information.
To the extent that any disruption or security breach were to result in a loss of, or damage to, our or our third- party vendors’, collaborators’ or other contractors’ or consultants’ data or applications, or inappropriate disclosure of confidential, personal or proprietary information, we could incur liability including litigation exposure, penalties and fines, we could become the subject of regulatory action or investigation, our competitive position could be harmed and the further development and commercialization of our product candidates could be delayed. Any of the above could have a material adverse effect on our business, financial condition, results of operations or prospects.
Our operations are vulnerable to interruption by fire, earthquakes, power loss, telecommunications failure, terrorist activity, pandemics and other events beyond our control, which could harm our business.
Our facilities are located in California. We have not undertaken a systematic analysis of the potential consequences to our business and financial results from a major flood, fire, earthquake, power loss, terrorist activity, pandemics or other disasters and do not have a recovery plan for such disasters. In addition, we do not carry sufficient insurance to compensate us for actual losses from interruption of our business that may occur, and any losses or damages incurred by us could harm our business. The occurrence of any of these business disruptions could seriously harm our operations and financial condition and increase our costs and expenses.
Risks Related to Ownership of Our Common Stock
We do not know whether a market will develop for our common stock or what the market price of our common stock will be, and, as a result, it may be difficult for you to sell your shares of our common stock.
Although our common stock is listed on the Nasdaq Global Market, an active trading market for our common stock may not be sustained. If a market for our common stock is not sustained, it may be difficult for you to sell your shares of common stock at an attractive price or at all. We cannot predict the prices at which our common stock will trade in the future. It is possible that in one or more future periods our results of operations may be below the expectations of public market analysts and investors, and, as a result of these and other factors, the price of our common stock may fall.
The market price of our common stock has been and may continue to be volatile and the value of an investment in our common stock may decline, which could result in substantial losses for investors.
The market price of our common stock has been and is likely to continue to be highly volatile. From February 7, 2022 through February 6, 2023, the trading price of our common stock ranged between a low sales price of $1.85 and a high sales price of $11.02. As
95
a result of this volatility, a holder may not be able to sell our common stock at or above the price at which such holder acquired shares of our common stock.
The market price for our common stock may be influenced by those factors discussed in this “Risk Factors” section and many others, including:
In recent years, the stock market in general, and the market for pharmaceutical and biotechnology companies in particular, has experienced extreme price and volume fluctuations that have often been unrelated or disproportionate to changes in the operating performance of the companies whose stock is experiencing those price and volume fluctuations. Broad market and industry factors may seriously affect the market price of our common stock, regardless of our actual operating performance. Following periods of such volatility in the market price of a company’s securities, securities class action litigation has often been brought against that company. Because of the potential volatility of our stock price, we may become the target of securities litigation in the future. Securities litigation could result in substantial costs and divert management’s attention and resources from our business.
A significant portion of our total outstanding shares may be sold into the market in the near future, which could cause the market price of our common stock to decline significantly.
Sales of a substantial number of shares of our common stock in the public market could occur at any time. These sales, or the perception in the market that the holders of a large number of shares of common stock intend to sell shares, could reduce the market price of our common stock. Persons who were our stockholders prior to our initial public offering continue to hold a substantial number of shares of our common stock. Significant portions of these shares are held by a small number of stockholders. Sales by our stockholders of a substantial number of shares, or the expectation that such sales may occur, could significantly reduce the market price of our common stock. Moreover, certain shares of our common stock have rights, subject to conditions, to require us to file registration statements covering their shares or to include their shares in registration statements that we may file for ourselves or other stockholders. We have also registered or intend to register all shares of common stock that we may issue under our equity compensation plans or that
96
are issuable upon exercise of outstanding options. These shares can be freely sold in the public market upon issuance and once vested, subject to volume limitations applicable to affiliates. In addition, our directors, executive officers and certain affiliates may establish programmed selling plans under Rule 10b5-1 of the Exchange Act for the purpose of effecting sales of our common stock. If any of these events cause a large number of our shares to be sold, or if it is perceived that they will be sold, in the public market, the market price of our common stock could decline.
Our principal stockholders and management own a significant percentage of our stock and will be able to exert significant control over matters subject to stockholder approval.
As of December 31, 2022, our executive officers, directors, holders of 5% or more of our capital stock and their respective affiliates beneficially owned approximately 63.4% of our common stock. This group of stockholders has the ability to control us through this ownership position. These stockholders may be able to determine all matters requiring stockholder approval. For example, these stockholders may be able to control elections of directors, amendments of our organizational documents or approval of any merger, sale of assets or other major corporate transaction. This may prevent or discourage unsolicited acquisition proposals or offers for our common stock that you may feel are in your best interest as one of our stockholders. The interests of this group of stockholders may not always coincide with the interests of our other stockholders and they may act in a manner that advances their best interests and not necessarily those of other stockholders, including seeking a premium value for their common stock, and might affect the prevailing market price for our common stock.
We are an “emerging growth company” and a “smaller reporting company,” and the reduced disclosure requirements applicable to emerging growth companies and smaller reporting companies may make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the JOBS Act and may remain an emerging growth company for up to five years. For so long as we remain an emerging growth company, we are permitted and plan to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include not being required to comply with the auditor attestation requirements of Section 404 of the Sarbanes-Oxley Act of 2002, as amended (“SOX”), not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation or a supplement to the auditor’s report providing additional information about the audit and the financial statements, reduced disclosure obligations regarding executive compensation, and exemptions from the requirements of holding a nonbinding advisory vote on executive compensation and stockholder approval of any golden parachute payments not previously approved. As a result, the information we provide stockholders will be different than the information that is available with respect to other public companies.
In addition, the JOBS Act provides that an emerging growth company can take advantage of an extended transition period for complying with new or revised accounting standards. This allows an emerging growth company to delay the adoption of certain accounting standards until those standards would otherwise apply to private companies. Accordingly, the information contained in our disclosure may be different from the information you receive from other public companies in which you hold stock. We have elected to use this extended transition period for complying with new or revised accounting standards that have different effective dates for public and private companies until the earlier of the date we (i) are no longer an emerging growth company or (ii) affirmatively and irrevocably opt out of the extended transition period provided in the JOBS Act. As a result, our financial statements may not be comparable to companies that comply with new or revised accounting pronouncements as of public company effective dates.
We are also a “smaller reporting company,” meaning that the market value of our stock held by non-affiliates is less than $700.0 million and our annual revenue is less than $100.0 million during the most recently completed fiscal year. We may continue to be a smaller reporting company if either (i) the market value of our stock held by non-affiliates is less than $250.0 million or (ii) our annual revenue is less than $100.0 million during the most recently completed fiscal year and the market value of our stock held by non-affiliates is less than $700.0 million. If we are a smaller reporting company at the time we cease to be an emerging growth company, we may continue to rely on exemptions from certain disclosure requirements that are available to smaller reporting companies. Specifically, as a smaller reporting company we may choose to present only the two most recent fiscal years of audited financial statements in our Annual Report on Form 10-K and, similar to emerging growth companies, smaller reporting companies have reduced disclosure obligations regarding executive compensation.
We cannot predict whether investors will find our common stock less attractive if we rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less active trading market for our common stock, and our stock price may be more volatile.
We have broad discretion in the use of the capital we have raised and may not use it effectively.
We cannot specify with certainty the particular uses of the capital we have raised, including the net proceeds from our initial public offering. Accordingly, you will have to rely upon the judgment of our management with respect to the use of these funds, with only limited information concerning management’s specific intentions. Our management may spend a portion or all of the net proceeds from our prior financings, including our initial public offering in ways that our stockholders may not desire or that may not yield a favorable return. The failure by our management to apply these funds effectively could harm our business, financial condition, results
97
of operations and prospects. Pending their use, we may invest the net proceeds from our prior financings, including our initial public offering in a manner that does not produce income or that loses value.
We do not expect to pay any dividends for the foreseeable future. Investors in our common stock may never obtain a return on their investment.
You should not rely on an investment in our common stock to provide dividend income. We do not anticipate that we will pay any dividends to holders of our common stock in the foreseeable future. Instead, we plan to retain any earnings to maintain and expand our existing operations. In addition, any future credit facility may contain terms prohibiting or limiting the amount of dividends that may be declared or paid on our common stock. Accordingly, investors must rely on sales of their common stock after price appreciation, which may never occur, as the only way to realize any return on their investment. As a result, investors seeking cash dividends should not purchase our common stock.
Provisions in our amended and restated certificate of incorporation, our amended and restated bylaws and Delaware law may have anti-takeover effects that could discourage an acquisition of us by others, even if an acquisition would be beneficial to our stockholders, and may prevent attempts by our stockholders to replace or remove our current management, which could depress the trading price of our common stock.
Our amended and restated certificate of incorporation, amended and restated bylaws and Delaware law contain provisions that may have the effect of discouraging, delaying or preventing a change in control of us or changes in our management that stockholders may consider favorable, including transactions in which you might otherwise receive a premium for your shares. Our amended and restated certificate of incorporation and bylaws include provisions that:
These provisions, alone or together, could delay or prevent hostile takeovers and changes in control or changes in our management. These provisions could also limit the price that investors might be willing to pay in the future for shares of our common stock, thereby depressing the market price of our common stock.
In addition, because we are incorporated in the State of Delaware, we are governed by the provisions of Section 203 of the General Corporation Law of the State of Delaware (DGCL), which prohibits a person who owns in excess of 15% of our outstanding voting stock from merging or combining with us for a period of three years after the date of the transaction in which the person acquired in excess of 15% of our outstanding voting stock, unless the merger or combination is approved in a prescribed manner.
Any provision of our amended and restated certificate of incorporation, amended and restated bylaws or Delaware law that has the effect of delaying or deterring a change in control could limit the opportunity for our stockholders to receive a premium for their shares of our common stock, and could also affect the price that some investors are willing to pay for our common stock.
Our amended and restated bylaws designate the Court of Chancery of the State of Delaware as the exclusive forum for certain state law litigation that may be initiated by our stockholders and the U.S. federal district courts as the exclusive forum for certain securities law actions, which could limit our stockholders’ ability to litigate disputes with us in a different judicial forum and increase the costs for our stockholders to pursue certain claims against us.
Pursuant to our amended and restated bylaws, unless we consent in writing to the selection of an alternative forum, the Court of Chancery of the State of Delaware will be the sole and exclusive forum for the following types of actions or proceedings under Delaware statutory or common law: (i) any derivative action or proceeding brought on our behalf; (ii) any action asserting a claim of breach of a
98
fiduciary duty owed by any of our current or former directors, officers or employees to us or our stockholders; (iii) any action asserting a claim arising pursuant to any provision of the DGCL, our amended and restated certificate of incorporation or our amended and restated bylaws (including their interpretation, validity or enforceability); or (iv) any action asserting a claim governed by the internal affairs doctrine. This exclusive forum provision will not apply to any causes of action arising under the Securities Act or the Exchange Act or any other claim for which the federal courts have exclusive jurisdiction. Unless we consent in writing to the selection of an alternate forum, the United States federal district courts shall be the sole and exclusive forum for resolving any complaint asserting a cause of action arising under the Securities Act. In addition, our amended and restated bylaws provide that any person or entity purchasing or otherwise acquiring any interest in shares of our capital stock is deemed to have notice of and consented to these exclusive forum provisions. The forum selection provisions in our amended and restated bylaws may limit our stockholders’ ability to litigate disputes with us in a judicial forum that they find favorable for disputes with us or our directors, officers or employees, which may discourage the filing of lawsuits against us and our directors, officers and employees, even though an action, if successful, might benefit our stockholders. In addition, these forum selection provisions may impose additional litigation costs for stockholders who determine to pursue any such lawsuits against us.
General Risk Factors
If securities analysts do not publish research or reports about our business or if they publish negative evaluations of our stock, the price of our stock could decline.
The trading market for our common stock will rely in part on the research and reports that industry or financial analysts publish about us or our business. If one or more of the analysts covering our business downgrade their evaluations of our stock, the price of our stock could decline. If one or more of these analysts cease to cover our stock, we could lose visibility in the market for our stock, which in turn could cause our stock price to decline.
If we fail to establish and maintain proper and effective internal control over financial reporting, investors may lose confidence in the accuracy and completeness of our financial reports and the market price of our common stock could decline significantly.
Ensuring that we have adequate internal financial and accounting controls and procedures in place so that we can produce accurate financial statements on a timely basis is a costly and time-consuming effort that needs to be re-evaluated frequently. As a public company, we are required to maintain internal controls over financial reporting and to report any material weaknesses in such internal controls. Section 404 of SOX requires annual management assessment of the effectiveness of our internal control over financial reporting. However, our auditors will not be required to formally attest to the effectiveness of our internal control over financial reporting pursuant to Section 404 of SOX until we are no longer an emerging growth company if we continue to take advantage of the exemptions available to us through the JOBS Act.
Implementing any appropriate changes to our internal controls may distract our officers and employees, entail substantial costs to modify our existing processes and take significant time to complete. These changes may not, however, be effective in maintaining the adequacy of our internal controls, and any failure to maintain that adequacy, or consequent inability to produce accurate financial statements on a timely basis, could increase our operating costs and harm our business. In addition, investors’ perceptions that our internal controls are inadequate or that we are unable to produce accurate financial statements on a timely basis could cause investors to lose confidence in the accuracy and completeness of our financial reports and could cause the market price of our common stock to decline significantly.
We will incur increased costs as a result of operating as a public company, and our management will be required to devote substantial time to new compliance initiatives and corporate governance practices.
As a public company, and particularly after we are no longer an “emerging growth company,” we will incur significant legal, accounting, and other expenses that we did not incur as a private company. SOX, the Dodd- Frank Wall Street Reform and Consumer Protection Act, the listing requirements of The Nasdaq Global Market, and other applicable securities rules and regulations impose various requirements on public companies, including establishment and maintenance of effective disclosure and financial controls and corporate governance practices. We expect that we will need to hire additional accounting, finance, and other personnel in connection with our efforts to comply with the requirements of being a public company, and our management and other personnel will need to devote a substantial amount of time towards maintaining compliance with these requirements. These requirements will increase our legal and financial compliance costs and will make some activities more time-consuming and costly. For example, the rules and regulations applicable to us as a public company make it more difficult and more expensive for us to maintain director and officer liability insurance. We are currently evaluating these rules and regulations and cannot predict or estimate the amount of additional costs we may incur or the timing of such costs. These rules and regulations are often subject to varying interpretations, in many cases due to their lack of specificity, and, as a result, their application in practice may evolve over time as new guidance is provided by regulatory and governing bodies. This could result in continuing uncertainty regarding compliance matters and higher costs necessitated by ongoing revisions to disclosure and governance practices.
Pursuant to Section 404 of SOX, we will be required to furnish a report by our management on our internal control over financial reporting beginning with our second filing of an Annual Report on Form 10-K with the SEC. However, while we remain an emerging
99
growth company, we will not be required to include an attestation report on internal control over financial reporting issued by our independent registered public accounting firm. To achieve compliance with Section 404 of SOX within the prescribed period, we will be engaged in a process to document and evaluate our internal control over financial reporting, which is both costly and challenging. In this regard, we will need to continue to dedicate internal resources, potentially engage outside consultants, adopt a detailed work plan to assess and document the adequacy of internal control over financial reporting, continue steps to improve control processes as appropriate, validate through testing that controls are functioning as documented, and implement a continuous reporting and improvement process for internal control over financial reporting. Despite our efforts, there is a risk that we will not be able to conclude, within the prescribed timeframe or at all, that our internal control over financial reporting is effective as required by Section 404 of SOX. If we identify one or more material weaknesses, it could result in an adverse reaction in the financial markets due to a loss of confidence in the reliability of our financial statements.
Our disclosure controls and procedures may not prevent or detect all errors or acts of fraud.
We designed our disclosure controls and procedures to reasonably assure that information we must disclose in reports we file or submit under the Exchange Act is accumulated and communicated to management, and recorded, processed, summarized and reported within the time periods specified in the rules and forms of the SEC. We believe that any disclosure controls and procedures or internal controls and procedures, no matter how well-conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met.
These inherent limitations include the facts that judgments in decision-making can be faulty and that breakdowns can occur because of simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by an unauthorized override of the controls. Accordingly, because of the inherent limitations in our control system, misstatements due to error or fraud may occur and not be detected.
Global economic uncertainty and unfavorable global economic conditions caused by political instability, changes in trade agreements and conflicts, such as the conflict between Russia and Ukraine, could adversely affect our business, financial condition, results of operations or prospects.
Our business, financial condition, results of operations or prospects could be adversely affected by general conditions in the global economy and in the global financial markets. A severe or prolonged economic downturn, economic uncertainties in various global markets caused by political instability and conflict, or additional global financial crises, could result in a variety of risks to our business, including weakened demand for our product candidates, if approved, or our inability to raise additional capital when needed on acceptable terms, if at all. A weak or declining economy could also strain our suppliers, possibly resulting in supply disruption. Any of the foregoing could harm our business and we cannot anticipate all of the ways in which the current economic climate and financial market conditions could adversely impact our business.
Unfavorable global economic conditions could adversely affect our business, financial condition or results of operations.
Our results of operations could be adversely affected by general conditions in the global economy and in the global financial markets. For example, rising interest rates and high inflation may cause our cost of doing business to materially increase and may adversely impact our ability to operate or may adversely impact other parties upon whom we rely for research and development capabilities to operate. The most recent global financial crisis caused extreme volatility and disruptions in the capital and credit markets. A severe or prolonged economic downturn, such as the most recent global financial crisis, could result in a variety of risks to our business, including weakened demand for our product candidates and our ability to raise additional capital when needed on acceptable terms, if at all. A weak or declining economy could strain our suppliers, possibly resulting in supply disruption, or cause delays in payments for our services by third-party payors or our collaborators. Any of the foregoing could harm our business and we cannot anticipate all of the ways in which the current economic climate and financial market conditions could adversely impact our business.
100
Item 1B. Unresolved Staff Comments.
None.
Item 2. Properties.
We lease 19,195 and 18,325 square feet of office and laboratory space in South San Francisco and Brisbane, California, respectively, under leases that expire in March 2025 and December 2023, respectively.
In addition, in late 2021, we entered into a non-cancelable operating lease for approximately 85,165 square feet in South San Francisco, California, which will commence in 2023 and expire in 2033. This property is currently available to sublease as we continue consolidating during the year ended December 31, 2023.
We believe that our facilities are sufficient to meet our current needs. Information as to material lease commitments is included in Financial Note 8, “Operating Leases,” to the financial statements appearing in this Annual Report on Form 10-K.
Item 3. Legal Proceedings.
We are not party to any material legal proceedings at this time. From time to time, we may become involved in various legal proceedings that arise in the ordinary course of our business.
Item 4. Mine Safety Disclosures.
Not applicable.
101
PART II
Item 5. Market for the Registrant's Common Equity, Related Stockholder Matters and Issuer Purchases of Equity Securities.
Market Information
Our common stock trades on the Nasdaq Global Select Market under the symbol “GRPH.” Trading of our common stock commenced on June 25, 2021 in connection with our initial public offering. Prior to that time, there was no established public trading market for our common stock.
Holders
As of March 14, 2023, we had approximately 32 holders of record of our common stock. This number does not include beneficial owners whose shares were held in street name.
Dividend Policy
We have never declared or paid any cash dividends on our common stock. We currently intend to retain future earnings to fund the development and growth of our business. We do not expect to pay any cash dividends in the foreseeable future. In addition, the terms of any future debt agreements that we may enter into may preclude us from paying dividends without the lenders’ consent or at all.
Securities Authorized for Issuance Under Equity Compensation Plans
The information required by Item 5 of Form 10-K regarding equity compensation plans is incorporated herein by reference to Item 12 of Part III of this Annual Report.
Recent Sales of Unregistered Securities
None.
Use of Proceeds from the Initial Public Offering of Common Stock
On June 29, 2021, we completed our IPO and issued 14,000,000 shares of our common stock at an initial offering price of $17.00 per share. On July 2, 2021, we issued 2,100,000 shares of our common stock to the underwriters of the IPO pursuant to the exercise of their option to purchase additional shares at a price of $17.00 per share less underwriting discounts and commissions. We received net proceeds from the IPO of approximately $251.3 million, after deducting underwriting discounts and commissions of approximately $19.1 million and offering expenses of approximately $3.2 million. None of the expenses associated with the IPO were paid to directors, officers, persons owning 10% or more of any class of equity securities, or to their associates. Morgan Stanley & Co. LLC, BofA Securities, Inc., Cowen and Company, LLC and SVB Leerink, LLC acted as book-running managers for the IPO.
Shares of our common stock began trading on The Nasdaq Global Market on June 25, 2021. The offer and sale of the shares were registered under the Securities Act on a registration statement on Form S-1 (Registration No. 333-256838), which was declared effective on June 24, 2021.
As of December 31, 2022, we have used approximately $129.9 million of the net proceeds received in the IPO. Cash used since the IPO is described elsewhere in the “Management’s Discussion and Analysis of Financial Condition and Results of Operations” section of our periodic reports filed with the SEC. There has been no material change in the planned use of proceeds from our IPO as described in the registration statement on Form S-1. We invested the funds received in cash equivalents and other marketable securities in accordance with our investment policy.
102
Purchases of Equity Securities by the Issuer and Affiliated Purchasers
The following table provides stock repurchase activity during each of the fourth quarter of the year ended December 31, 2022:
|
|
Total number of shares purchased(1) |
|
|
|
Average price paid per share |
|
|
Total number of shares purchased as part of publicly announced plans or programs |
|
|
Maximum number of shares that may yet be purchased under the plans or programs |
|
||||
October 1, 2022 - October 31, 2022 |
|
|
— |
|
|
$ |
|
— |
|
|
|
— |
|
|
|
— |
|
November 1, 2022 - November 30, 2022 |
|
|
1,507 |
|
|
|
|
0.30 |
|
|
|
— |
|
|
|
— |
|
December 1, 2022 - December 31, 2022 |
|
|
58,319 |
|
|
|
|
0.30 |
|
|
|
— |
|
|
|
— |
|
Total |
|
|
59,826 |
|
|
$ |
|
0.30 |
|
|
|
— |
|
|
|
— |
|
Item 6. Reserved.
103
Item 7. Management’s Discussion and Analysis of Financial Condition and Results of Operations.
Management’s discussion and analysis of financial condition and results of operations, referred to as the “Financial Review,” is intended to assist the reader in the understanding and assessment of significant changes and trends related to the results of operations and financial position of Graphite Bio ( the “Company,” “Graphite,” “we,” “our,” or “us” and other similar pronouns). This discussion and analysis should be read in conjunction with the financial statements and accompanying financial notes in Item 8 of Part II of this Annual Report on Form 10-K.
Certain statements in this report constitute forward-looking statements. See Cautionary Note Regarding Forward-Looking Statements of this Annual Report on Form 10-K for additional factors relating to these statements and Item 1A - Risk Factors in Part I of this Annual Report on Form 10-K for a list of certain risk factors applicable to our business, financial condition, and results of operations.
Overview
We are a clinical-stage, next-generation gene editing company harnessing high-efficiency targeted gene integration to develop a new class of therapies to potentially cure a wide range of serious and life-threatening diseases. Our precision gene editing approach aims to achieve one of medicine’s most elusive goals: to precisely “find & replace” any gene in the genome. We have a next-generation gene editing platform that is designed to precisely correct mutations, replace entire disease-causing genes with normal genes, or insert new genes into predetermined, safe locations. We believe our approach could enable broad applications to transform human health, including directly correcting mutations, engineering cells to permanently deliver therapeutic proteins, and precisely engineering effector cells to treat or cure a wide range of serious genetic and other diseases, including cancer, autoimmune and neurodegenerative diseases.
Our lead product candidate nulabeglogene autogedtemcel (nula-cel), formerly GPH101, is a highly differentiated approach with the potential to directly correct the mutation that causes SCD and restore normal HgbA expression. Curing sickle cell disease by correcting the disease-causing point mutation to normal is viewed as the gold-standard for curing SCD and has been the dream of treating physicians for generations. In January 2023, we announced a voluntary pause of our Phase 1/2 CEDAR study of nula-cel for sickle cell disease due to a serious adverse event in the first patient dosed, which we concluded is likely related to study treatment. In February 2023, we announced our decision to discontinue the development of nula-cel and initiate a process to explore strategic alternatives.
We were incorporated in Ontario, Canada in June 2017 as Longbow Therapeutics Inc. and were reincorporated in the State of Delaware in October 2019. In February 2020, we changed our name to Integral Medicines, Inc. and in August 2020, we changed our name to Graphite Bio, Inc. Research and development of our initial technology ceased at the end of 2018 and we did not have any significant operations or any research and development activities in 2019. In March 2020, we identified new gene editing technology which we sought to further develop, and we licensed the related intellectual property rights from The Board of Trustees of the Leland Stanford Junior University (“Stanford”) in December 2020.
Since our inception in June 2017, we have devoted substantially all of our resources to performing research and development, enabling manufacturing activities in support of our product development efforts, hiring personnel, acquiring and developing our technology and product candidates, organizing and staffing our Company, performing business planning, establishing our intellectual property portfolio, raising capital and providing general and administrative support for these activities. We have one product candidate that has an accepted IND. All of our other product candidates are in preclinical development, and we do not have any products approved for sale and have not generated any revenue from product sales. To date, we have funded our operations primarily with an aggregate of $197.7 million in aggregate gross proceeds from the sales of our redeemable convertible preferred stock and the issuance of convertible notes. In June and July 2021, we completed our initial public offering (“IPO”) and issued 16,100,000 shares of our common stock for $17.00 a share with a total net proceeds of approximately $251.3 million, and total underwriting costs of $19.1 million and issuance costs of $3.2 million. We will continue to require additional capital to develop our product candidates and fund operations for the foreseeable future. Accordingly, until such time as we can generate significant revenue from sales of our product candidates, if ever, we expect to finance our cash needs through public or private equity or debt financings, and collaborations, strategic alliances and licensing arrangements with third parties.
We have incurred significant operating losses since inception. As of December 31, 2022, we had cash and cash equivalents and investments in marketable securities of $283.6 million and an accumulated deficit of $242.4 million. We expect to continue to incur substantial losses for the foreseeable future, and our transition to profitability will depend upon successful development, approval and commercialization of our product candidates and upon achievement of sufficient revenues to support our cost structure. We do not expect to generate any revenue from commercial product sales unless and until we successfully complete development and obtain regulatory approval for one or more of our product candidates, which we expect will take at least several years. We may never achieve profitability, and unless and until then, we will need to continue to raise additional capital. Based upon our current operating plan, we estimate that our cash, cash equivalents and restricted cash as of December 31, 2022 will be sufficient to fund our operating expenses and capital expenditure requirements for at least the next 12 months.
104
We expect our expenses will increase substantially in connection with our ongoing and planned activities, as we:
We rely and will continue to rely on third parties in the conduct of our preclinical studies and clinical trials and for manufacturing and supply of our product candidates. We have no internal manufacturing capabilities, and we may continue to rely on third parties for our preclinical and clinical trial materials, of which the main suppliers are single-source suppliers. Given our stage of development, we do not yet have a marketing or sales organization or commercial infrastructure. Accordingly, if we obtain regulatory approval for any of our product candidates, we also expect to incur significant commercialization expenses related to product sales, marketing, manufacturing and distribution.
Because of the numerous risks and uncertainties associated with product development, we are unable to predict the timing or amount of increased expenses or when or if we will be able to achieve or maintain profitability. Even if we are able to generate revenue from sales of any product for which we receive regulatory approval, we may not become profitable. If we fail to become profitable or are unable to sustain profitability on a continuing basis, we may be unable to continue our operations at planned levels and may be forced to reduce our operations.
Stanford Exclusive License Agreement and Option Agreement
In December 2020, we entered into an exclusive license agreement (the “License Agreement”), with The Board of Trustees of the Leland Stanford Junior University (Stanford), pursuant to which Stanford granted us a worldwide license to specified technology and patent rights to develop, manufacture and commercialize human prophylactic and therapeutic products. Other than with respect to specified, broadly applicable assays and procedures and subject to retained rights by Stanford, the license is exclusive with respect to human prophylactic and therapeutic products for the treatment of SCD, XSCID and beta thalassemia. The license is non-exclusive with respect to those broadly applicable assays and procedures and with respect to all human prophylactic and therapeutic products other than for the treatment of SCD, XSCID and beta thalassemia.
To date, pursuant to the License Agreement, we have paid an upfront license fee to Stanford of $50.0 thousand and issued to Stanford and its designees an aggregate of approximately 0.6 million shares of our common stock. The acquisition of the exclusive license, including patent rights and know-how, and clinical supplies was accounted for as an asset acquisition and as the acquired technology and inventories did not have an alternative use, the total consideration of $2.8 million was recorded as research and development expense in the statements of operations and comprehensive loss for the year ended December 31, 2020. We are obligated to pay Stanford an annual license maintenance fee on each anniversary of the effective date of the License Agreement. The annual license maintenance fee initially is $5.0 thousand and will increase to $50.0 thousand in three increments over the first seven anniversaries of the effective date of the License Agreement. After the first commercial sale of a product falling within the scope of the license (the “Licensed Product”), the annual license maintenance fee is $200.0 thousand.
In May 2021, we issued 640,861 shares of our common stock in connection with the License Agreement. Subsequently, in June 2021, related to the License Agreement, we repurchased 624,845 shares of our common stock from investors and founders.
We are required to share with Stanford a portion of any non-royalty income we receive from sublicensing the licensed patent rights or technology, subject to specified exclusions. With respect to sublicenses granted to products for the treatment of SCD, XSCID and beta thalassemia, the portion of sublicense income we must share with Stanford varies by indication and declines from between a mid-teen to a second quartile double-digit percentage prior to the filing of an IND to between a high single-digit to very low double-digit percentage upon achievement of a specified clinical milestone. With respect to sublicenses granted under the licensed technology rights and not licensed patent rights, the portion of sublicense income shared with Stanford declines from between a mid-single-digit and very low double-digit percentage prior to the filing of an IND to a low single-digit percentage after filing of an IND.
105
We are obligated to make payments to Stanford with respect to each Licensed Product of up to an aggregate of $12.8 million upon the achievement of certain development, regulatory and commercial milestones. Such amounts are payable only once upon the first occurrence of a particular milestone event with respect to each Licensed Product and only once with respect to each new indication covered by any of the Licensed Products.
We also are obligated to pay Stanford low single-digit royalties based on worldwide annual net sales of any Licensed Product, subject to specified reductions. We will be obligated to continue to pay royalties on a Licensed Product-by-Licensed Product and country-by-country basis, until the latest of (i) the expiration of the last valid claim under the licensed patents that covers the sale or manufacture of such Licensed Product in such country, (ii) the expiration of any period of regulatory exclusivity with respect to such Licensed Product in such country or (iii) the expiration of ten years after the first commercial sale of such Licensed Product in such country.
The term of the License Agreement expires on the later of (i) the expiration of the last patent or abandonment of the last patent application within the license patent rights or (ii) the expiration of all royalty terms with respect to Licensed Products. The License Agreement may be terminated by us at will or by Stanford if we remain in breach of the License Agreement following a cure period to remedy the breach.
We are required to use diligent efforts to manufacture, market and sell Licensed Products for the treatment of each of SCD, XSCID and beta thalassemia. In addition, we are required to achieve specified milestones by specified dates with respect to Licensed Products for the treatment of each of SCD, XSCID and beta thalassemia. If we fail to satisfy our diligence obligations, Stanford may terminate the License Agreement for our breach. For more details on the License Agreement, please see Note 5 of the Notes to Financial Statements.
In January 2021, we entered into an option agreement (the “First Option Agreement”), with Stanford, pursuant to which Stanford granted us the right to obtain a license to specified patent rights relating to human prophylactic and therapeutic products. We may exercise the option in whole or in part to obtain a license under one or more of the optioned patent rights.
Subject to our exercise of the option under the First Option Agreement and our execution of an amendment to the License Agreement that incorporates the optioned patent rights and any optioned technology, we have agreed to issue to Stanford 132,137 shares of our common stock and pay a license execution fee of $10.0 thousand. The term of the First Option Agreement expires 18 months after its effective date, subject to our right to extend such expiration date by up to an additional one year upon notice to Stanford and by another additional one year upon the reasonable agreement of Stanford. The First Option Agreement will terminate if the License Agreement terminates. On June 23, 2022, the Company exercised its right to extend the term of the First Option Agreement for an additional year. As of March 1, 2023, the Company had not exercised the option under the First Option Agreement and no fees have been paid for the First Option Agreement.
In April 2021, we entered into an option agreement (the “Second Option Agreement”) with Stanford to negotiate the license for additional technologies from Stanford. Pursuant to the Second Option Agreement, we agreed to pay Stanford option fees in an aggregate amount of $30.0 thousand over the term of the option. On April 13, 2022, we entered into an amendment to the Second Option Agreement which extended the term for an additional year and the maintenance fee of $10.0 thousand was paid during the year ended December 31, 2022. As of March 1, 2023, we have not exercised the option and no fees have been paid under the Second Option Agreement.
LCGM Service Agreement
On August 30, 2021, we entered into a Master Manufacturing and Service Agreement with the Laboratory for Cell & Gene Medicine (“LCGM”) at Stanford (“LCGM MSA”). Pursuant to the LCGM MSA, LCGM will conduct clinical manufacturing, release testing, and product release for nula-cel in our Phase 1 clinical trial to treat SCD. During 2021, we entered into various SOWs under the LCGM MSA under which we received technology transfer and related services for HBB Beta-Globin Gene Variant for SCD, manufacturing engineer test runs, the exclusive use of a manufacturing suite at the LCGM facility, and Phase 1/2 clinical development and manufacturing of the HBB Variant for SCD. We have recognized $6.1 and $2.7 million in research and development expense in connection with the LCGM MSA during the years ended December 31, 2022 and 2021, respectively.
IDT License Agreement
On June 7, 2021, we entered into a License Agreement (the “IDT License Agreement”) with Integrated DNA Technologies, Inc. (IDT). Pursuant to the IDT License Agreement, IDT granted us and our affiliates a worldwide, non-exclusive, sublicensable license to research and develop products incorporating HiFi Cas9 protein variants for use in human therapeutic applications for SCD, XSCID and Gaucher disease (the “Field”) and a worldwide, exclusive, sublicensable license to commercialize such products in the Field. We have also been granted the right to expand the licensed Field to include human therapeutic applications in the additional fields of beta thalassemia disorder and lysosomal storage disorders upon the payment of an exercise fee in the amount of $0.5 million per additional field or $1.0 million for both additional fields.
In consideration of the licenses and rights granted to us under the IDT License Agreement, we agreed to pay to IDT an upfront payment in the amount of $3.0 million and up to $5.3 million (or $8.8 million if we elect to expand the Field as described above to
106
include both the beta thalassemia and lysosomal storage disorders fields) in total regulatory milestone payments. Each regulatory milestone payment is payable once on an indication-by-indication basis. In addition, we have agreed to pay IDT a low single-digit royalty on the net sales of products, subject to reductions in specified circumstances. The acquisition of the license was accounted for as an asset acquisition and as the acquired technology did not have an alternative use, the total consideration of $3.0 million was recorded as research and development expense in the statement of operations and comprehensive loss for the December 31, 2021. During the year ended December 31, 2022, we have not recognized any research and development expense in connection with the IDT License Agreement. There are no milestones probable as of December 31, 2022; therefore, no milestone payments have been recognized in during the year ended December 31, 2022.
The IDT License Agreement remains in effect on a country-by-country and product-by-product basis until the expiration of the royalty term for such product in such jurisdiction. We and IDT each have the right to terminate the IDT License Agreement for the other party’s material breach of its obligations under the IDT License Agreement, subject to specified rights to cure. Additionally, we may terminate the IDT License Agreement for any reason upon written notice.
Master Development and Manufacturing Services Agreement (for cell therapy services)
In November 2022, we entered into a master development and manufacturing services agreement, with WuXi Advanced Therapies, Inc. (WuXi) (the “WuXi Agreement”), pursuant to which we may engage WuXi, at our discretion, to provide services under separate work orders for cell therapy services. To date, pursuant to the WuXi Agreement, we have entered into three work orders for cell therapy services. Following our decision to discontinue the development of nula-cel, we do not intend to enter into new work orders under the WuXi Agreement.
Initial Public Offering
In June and July 2021, we completed an initial public offering of our common stock. As part of the IPO, we issued and sold 16,100,000 shares of our common stock at a public offering price of $17.00 per share. In June and July 2021, we received net proceeds of approximately $251.3 million from the IPO, after deducting underwriting discounts and commissions of $19.1 million and offering costs of approximately $3.2 million.
Components of Results of Operations
Operating Expenses
Research and Development
Research and development costs consist primarily of external and internal costs incurred for our research activities and the development of our gene editing platform and associated rights which we licensed in December 2020.
External costs include:
Research and development costs are expensed as incurred. The intellectual property we licensed in late 2020 is fundamental to our platform and we did not focus on any specific programs. In the future, we expect to track research and development costs on a program by program basis as we identify the specific programs and product candidates to develop.
During 2022 and 2021, we were eligible for a research and development tax credit. The tax incentive was available to us based on research and development activity within the United States and California during that year. These research and development tax incentives are recognized as a reduction to payroll tax expense when the right to receive has been attained and funds are collectible and are capped at $250.0 thousand per year.
We expect our research and development expenses to increase substantially for the foreseeable future as we advance our product candidates into and through preclinical studies and clinical trials, pursue regulatory approval of our product candidates and expand our
107
pipeline of product candidates. The process of conducting the necessary preclinical and clinical research to obtain regulatory approval is costly and time-consuming. The actual probability of success for our product candidates may be affected by a variety of factors, including the safety and efficacy of our product candidates, early clinical data, investment in our clinical programs, competition, manufacturing capability and commercial viability. We may never succeed in achieving regulatory approval for any of our product candidates. As a result of the uncertainties discussed above, we are unable to determine the duration and completion costs of our research and development projects or if, when and to what extent we will generate revenue from the commercialization and sale of our product candidates, if approved by the FDA and other applicable authorities.
Our future research and development costs may vary significantly based on factors such as:
General and Administrative Expenses
General and administrative expenses consist primarily of expenses related to employee-related costs, including salaries, benefits and stock-based compensation expense, for our executive, business development, finance and accounting, human resources and other administrative functions; legal services, including relating to intellectual property and corporate matters; accounting, auditing, consulting and tax services; insurance; and facility and other allocated costs not otherwise included in research and development expenses. We expect our general and administrative expenses to increase substantially for the foreseeable future as we anticipate an increase in our personnel headcount to support expansion of research and development activities, as well as to support our operations generally. We also expect an increase in expenses associated with being a public company, including costs related to accounting, audit, legal, regulatory, and tax-related services associated with maintaining compliance with applicable Nasdaq and SEC requirements; director and officer insurance costs; and investor and public relations costs.
Other Income (Expense), Net
Other income (expense), net includes amounts realized from interest income from the investments in marketable securities and changes in the fair value of our redeemable convertible preferred stock tranche liabilities.
108
Results of Operations
Years Ended December 31, 2022 and 2021
The following table summarizes our statements of operations and comprehensive loss for the respective years (in thousands):
|
|
Year Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Operating expenses: |
|
|
|
|
|
|
||
Research and development |
|
$ |
72,787 |
|
|
$ |
37,932 |
|
General and administrative |
|
|
32,852 |
|
|
|
22,511 |
|
Total operating expenses |
|
|
105,639 |
|
|
|
60,443 |
|
Loss from operations |
|
|
(105,639 |
) |
|
|
(60,443 |
) |
Other income (expense), net: |
|
|
|
|
|
|
||
Interest income, net |
|
|
4,587 |
|
|
|
24 |
|
Change in fair value of the Series A redeemable convertible preferred stock tranche liability |
|
|
- |
|
|
|
(10,341 |
) |
Total other income (expense), net |
|
|
4,587 |
|
|
|
(10,317 |
) |
Net loss |
|
$ |
(101,052 |
) |
|
$ |
(70,760 |
) |
Unrealized loss on investments |
|
|
(1,048 |
) |
|
|
— |
|
Comprehensive loss |
|
$ |
(102,100 |
) |
|
$ |
(70,760 |
) |
Operating Expenses
Research and Development Expenses
Research and development expenses were $72.8 million for the year ended December 31, 2022 compared to $37.9 million for the year ended December 31, 2021, an increase of $34.9 million. The increase in research and development expenses was primarily attributable to an increase of $15.9 million in clinical trial related activities and contract manufacturing activities for our clinical trials and drug supply, $12.7 million of personnel costs, including associated stock-based compensation expense, and $6.3 million of other research and development costs primarily related to facilities costs and lease expense associated with facilities and service agreements.
General and Administrative Expenses
General and administrative expenses were $32.9 million for the year ended December 31, 2022 compared to $22.5 million for the year ended December 31, 2021, an increase of $10.4 million. The increase in general and administrative expenses was comprised of an increase of $7.3 million in personnel-related costs, including associated stock-based compensation expense. We also incurred other miscellaneous expenses costs of $1.9 million related to as well as professional expenses consisting of legal costs, accounting, financial service, and insurance costs of $1.2 million.
Other Income (Expense), Net
The other income (expense), net for the year ended December 31, 2022 comprised of interest income from the investments in marketable securities and income from money market funds. The other income (expense), net for the year ended December 31, 2021 was comprised of the change in the fair value of our Series A redeemable convertible preferred stock tranche liability of $10.3 million and income from money market funds. Investments in marketable securities were not held in the comparable period.
Liquidity and Capital Resources
We have incurred losses since inception and have incurred negative cash flows from operations from inception through December 31, 2022. As of December 31, 2022, we had $283.6 million of cash and cash equivalents and investments in marketable securities and our accumulated deficit was $242.4 million. In June and July 2021, we raised net proceeds of $251.3 million in our IPO, pursuant to which we sold an aggregate of 16,100,000 shares of common stock.
Prior to our IPO, we funded our operations primarily from the sale of redeemable convertible preferred stock and issuance of convertible promissory notes.
Future Funding Requirements
Our primary uses of cash are to fund our operations, which consist primarily of research and development expenditures related to our programs and, to a lesser extent, general and administrative expenditures. We anticipate that we will continue to incur significant expenses for the foreseeable future as we continue to advance our product candidates, expand our corporate infrastructure, including the costs associated with being a public company, further our research and development initiatives for our product candidates, scale our laboratory and manufacturing operations, and incur marketing costs associated with potential commercialization. We are subject to all
109
of the risks typically related to the development of new drug candidates, and we may encounter unforeseen expenses, difficulties, complications, delays and other unknown factors that may adversely affect our business. We anticipate that we will need substantial additional funding in connection with our continuing operations.
Based upon our current operating plan, we estimate that our existing cash, cash equivalents and investments in marketable securities as of the date of the filing of this Form 10-K, will be sufficient to fund our operating expenses and capital expenditure requirements into the fourth quarter of 2024. Until we can generate sufficient revenues from the commercialization of our product candidates or from collaboration agreements with third parties, if ever, we expect to finance our future cash needs through public or private equity or debt financings, collaborations and other strategic alliances and licensing arrangements, or any combination of these approaches. The sale of equity or convertible debt securities may result in dilution to our stockholders and, in the case of preferred equity securities or convertible debt, those securities could provide for rights, preferences or privileges senior to those of our common stock. Debt financings may subject us to covenant limitations or restrictions on our ability to take specific actions, such as incurring additional debt, making capital expenditures or declaring dividends. Our ability to raise additional funds may be adversely impacted by negative global economic conditions and any disruptions to and volatility in the credit and financial markets in the United States and worldwide that may result from the ongoing COVID-19 pandemic or other factors. There can be no assurance that we will be successful in acquiring additional funding at levels sufficient to fund our operations or on terms favorable or acceptable to us. If we are unable to obtain adequate financing when needed or on terms favorable or acceptable to us, we may be forced to delay, reduce the scope of or eliminate one or more of our research and development programs.
Our future capital requirements will depend on many factors, including:
A change in the outcome of any of these or other variables could significantly change the costs and timing associated with the development of our product candidates. Furthermore, our operating plans may change in the future, and we may need additional funds to meet operational needs and capital requirements associated with such change.
110
Cash Flows
The following table summarizes our sources and uses of cash for the periods presented (in thousands):
|
|
Year Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Net cash used in operating activities |
|
$ |
(87,980 |
) |
|
$ |
(52,852 |
) |
Net cash used in investing activities |
|
|
(241,863 |
) |
|
|
(5,740 |
) |
Net cash provided by financing activities |
|
|
597 |
|
|
|
417,467 |
|
Net increase (decrease) in cash, cash equivalents and restricted cash |
|
$ |
(329,246 |
) |
|
$ |
358,875 |
|
Cash Flows from Operating Activities
Net cash used in operating activities was $88.0 million for the year ended December 31, 2022, which was primarily attributable to our net loss of $101.1 million and net changes in operating assets and liabilities of $7.2 million, adjusted for net noncash charges of $20.3 million. Net noncash charges included approximately $13.5 million in stock-based compensation expense, $6.0 million in noncash lease expense, $2.4 million in depreciation and amortization expense, which was partially offset by $1.6 million in net amortization of premiums and discounts in marketable securities. Net changes in operating assets and liabilities primarily consists of $5.5 million in operating lease liabilities, $3.2 million in prepaid expenses, offset by $1.1 million in accrued compensation and $0.4 million in accounts payable and accrued expenses.
Net cash used in operating activities was $52.9 million for the year ended December 31, 2021, which was primarily attributable to our net loss of $70.8 million and net changes in operating assets and liabilities of $2.5 million, adjusted for net noncash charges of $20.4 million. Net noncash charges included approximately $10.3 million change in the fair value of the Series A redeemable convertible preferred stock tranche liability, $7.9 million in stock-based compensation expense, $1.5 million in noncash lease expense, and $0.7 million in depreciation and amortization expense. Net changes in operating assets and liabilities primarily consists of $2.2 million in accrued compensation and $1.0 million in accounts payable and accrued expenses, offset by $3.9 million in prepaid expenses and $1.8 million in operating lease liabilities.
Cash Flows from Investing Activities
Net cash used in investing activities was $241.9 million for the year ended December 31, 2022, which was primarily attributable to investment in current and non-current marketable securities of $405.5 million and the purchase of lab equipment of $6.6 million for use at our headquarters, offset by proceeds from our investments in current and non-current marketable securities of $170.2 million.
Net cash used in investing activities was $5.7 million for the year ended December 31, 2021, which was primarily attributable to the purchase of lab equipment for use at our headquarters.
Cash Flows from Financing Activities
Net cash provided by financing activities was $0.6 million for the year ended December 31, 2022, which consisted primarily of proceeds from issuance of common stock related to stock option grants and employee stock purchase plan of $0.7 million, which was partially offset by repurchases of unvested early exercised stock grants of $0.1 million.
Net cash provided by financing activities was $417.5 million for the year ended December 31, 2021, which consisted primarily of proceeds from issuance of common stock in our initial public offering of $251.3 million including proceeds from the exercise of the underwriter option in July 2021, proceeds from the issuance of Series A and Series B redeemable convertible preferred stock of $165.5 million, and proceeds from issuance of common stock related to early exercised stock options, stock option grants, and employee stock purchase plan of $0.7 million.
Recently Adopted Accounting Pronouncements
For information on new accounting standards, see Note 2 to our financial statements included in Part II in this Annual Report.
Critical Accounting Policies and Significant Judgments and Estimates
Our management’s discussion and analysis of our financial condition and results of operations are based on our financial statements, which have been prepared in accordance with accounting principles generally accepted in the United States of America. The preparation of these financial statements requires us to make estimates and judgments that affect the reported amounts of assets, liabilities, and expenses and the disclosure of contingent assets and liabilities in our financial statements. On an ongoing basis, we evaluate our estimates and judgments, including but not limited to those related to accrued research and development costs, the fair value of derivative redeemable convertible preferred stock tranche liabilities, the fair value of redeemable convertible preferred stock and common stock and stock-based compensation expense, the valuation of deferred tax assets, and uncertain income tax positions. We
111
base our estimates on historical experience, known trends and events and various other factors that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ from these estimates under different assumptions or conditions.
We believe that the accounting policies discussed below are critical to understanding our historical and future performance, as these policies relate to the more significant areas involving management’s judgments and estimates.
Accrued research and development expenses
As part of the process of preparing our financial statements, we estimate our accrued research and development expenses at each balance sheet date. This process involves reviewing purchase orders and open contracts, communicating with our personnel to identify services that have been performed on our behalf and estimating the level of service performed and the associated costs incurred for the services when we have not yet been invoiced or otherwise notified of the actual cost. The majority of our service providers invoice us monthly in arrears for services performed or when contractual milestones are met; however, some require advance payments. We make estimates of our accrued expenses as of each balance sheet date in our financial statements based on facts and circumstances known to us at that time. We periodically confirm the accuracy of our estimates with the service providers and make adjustments as necessary. The significant estimates in our accrued research and development expenses include the costs incurred for services performed by CROs and CMOs in connection with research and development activities for which we have not yet been invoiced.
We base our expenses related to research and development activities on our estimates of the services received and efforts expended pursuant to quotes and contracts with vendors that conduct research and development on our behalf. The financial terms of these agreements are subject to negotiation, vary from contract to contract and may result in uneven payment flows. There may be instances in which payments made to our vendors will exceed the level of services provided and result in a prepayment of the research and development expense. In accruing service fees, we estimate the time period over which services will be performed and the level of effort to be expended in each period. If the actual timing of the performance of services or the level of effort varies from our estimate, we adjust the accrual or prepaid accordingly. Non-refundable advance payments for goods and services that will be used in future research and development activities are expensed when the activity has been performed or when the goods have been received rather than when the payment is made.
Although we do not expect our estimates to be materially different from amounts incurred, our understanding of the status and timing of services performed relative to the actual status and timing of services performed may vary and may result in us reporting amounts that are too high or too low in any period. To date, there have been no material differences between our estimates of such expenses and the amounts incurred.
Stock-based compensation
We measure stock options and other stock-based awards granted to our employees, directors, consultants or founders based upon their fair value on the date of the grant and recognize stock-based compensation expense over the requisite service period, which is generally the vesting period of the respective award. We recognize forfeitures as they occur.
The majority of our stock-based compensation awards are subject to either service- or performance-based vesting conditions. We apply the straight-line method of expense recognition to all awards with service-based vesting and recognize stock-based compensation for performance-based awards over the service period using the accelerated attribution method to the extent achievement of the of performance condition is probable.
We estimate the fair value of each stock option grant on the date of grant using the Black-Scholes option-pricing model, which uses inputs such as the fair value of our common stock, assumptions we make for the volatility of our common stock, the expected term of our stock options, the risk-free interest rate for a period that approximates the expected term of our stock options and our expected dividend yield. The fair value of our common stock is used to determine the fair value of restricted stock awards.
Prior to our IPO in June 2021, there was no public market for our common stock. As a result, prior to our IPO, the estimated fair value of our common stock was determined by our board of directors as of the date of each option grant, with input from management, considering our most recently available third-party valuations of common stock and our board of directors’ assessment of additional objective and subjective factors that it believed were relevant and which may have changed from the date of the most recent valuation through the date of the grant. Following our IPO, the fair value of our common stock is determined based on the quoted market price of our common stock.
Emerging Growth Company and Smaller Reporting Entity Status
We are an emerging growth company, as defined in the JOBS Act. Under the JOBS Act, emerging growth companies can delay the adoption of new or revised accounting standards issued subsequent to the enactment of the JOBS Act until such time as those standards apply to private companies. Other exemptions and reduced reporting requirements under the JOBS Act for emerging growth companies include presentation of only two years of audited financial statements in a registration statement for an initial public offering, an exemption from the requirement to provide an auditor’s report on internal controls over financial reporting pursuant to Section 404 of the Sarbanes-Oxley Act of 2002, as amended, an exemption from any requirement that may be adopted by the Public Company
112
Accounting Oversight Board regarding mandatory audit firm rotation and less extensive disclosure about our executive compensation arrangements. We have elected to use the extended transition period for complying with new or revised accounting standards that have different effective dates for public and private companies until the earlier of the date that (i) we are no longer an emerging growth company or (ii) we affirmatively and irrevocably opt out of the extended transition period provided in the JOBS Act.
However, as described in Note 2 to our financial statements included elsewhere in this Annual Report, we early adopted certain accounting standards, as the JOBS Act does not preclude an emerging growth company from adopting a new or revised accounting standard earlier than the time that such standard applies to private companies to the extent early adoption is permitted. As a result, our financial statements may not be comparable to companies that comply with the new or revised accounting pronouncements as of public company effective dates.
We will remain an emerging growth company until the earliest of: (i) the last day of our first fiscal year in which we have total annual gross revenues of $1.23 billion or more, (ii) December 31, 2026, (iii) the date on which we are deemed to be a “large accelerated filer,” under the rules of the SEC, which means the market value of equity securities that is held by non-affiliates exceeds $700.0 million as of the prior June 30th and (iv) the date on which we have issued more than $1.0 billion in non-convertible debt securities during the prior three-year period.
If we are a “smaller reporting company” at the time we cease to be an emerging growth company, we may continue to rely on exemptions from certain disclosure requirements that are available to smaller reporting companies. Specifically, as a smaller reporting company we may choose to present only the two most recent fiscal years of audited financial statements in our Annual Report on Form 10-K and, similar to emerging growth companies, smaller reporting companies have reduced disclosure obligations regarding executive compensation.
113
Item 7A. Quantitative and Qualitative Disclosures about Market Risk.
Under SEC rules and regulations, as a smaller reporting company, we are not required to provide the information required by this item.
114
Item 8. Financial Statement and Other Supplementary Information.
Graphite Bio, Inc.
INDEX TO FINANCIAL STATEMENTS
Index to Audited Financial Statements as of and for the Years Ended December 31, 2022 and 2021: |
Page |
116 |
|
117 |
|
118 |
|
Statements of Redeemable Convertible Preferred Stock and Stockholders’ Deficit |
119 |
120 |
|
121 |
115
Report of Independent Registered Public Accounting Firm
To the Stockholders and the Board of Directors of Graphite Bio, Inc.
Opinion on the Financial Statements
We have audited the accompanying balance sheets of Graphite Bio, Inc. (the "Company") as of December 31, 2022 and 2021, the related statements of operations and comprehensive loss, redeemable convertible preferred stock and stockholders' deficit, and cash flows, for each of the two years in the period ended December 31, 2022, and the related notes (collectively referred to as the "financial statements"). In our opinion, the financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2022 and 2021, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2022, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
\These financial statements are the responsibility of the Company's management. Our responsibility is to express an opinion on the Company's financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion
.
/s/ Deloitte & Touche LLP
San Francisco, California
March 20, 2023
We have served as the Company’s auditor since 2021.
116
Graphite Bio, Inc.
Balance Sheets
(in thousands, except share and per share data)
|
|
December 31, |
|
|
December 31, |
|
||
|
|
2022 |
|
|
2021 |
|
||
|
|
|
|
|
|
|
||
Assets |
|
|
|
|
|
|
||
Current assets: |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
$ |
47,730 |
|
|
$ |
376,976 |
|
Investments in marketable securities, current |
|
|
220,499 |
|
|
|
— |
|
Prepaid expenses and other current assets |
|
|
7,136 |
|
|
|
4,760 |
|
Total current assets |
|
|
275,365 |
|
|
|
381,736 |
|
Restricted cash |
|
|
1,716 |
|
|
|
1,716 |
|
Investments in marketable securities, non-current |
|
|
15,322 |
|
|
|
— |
|
Property and equipment, net |
|
|
22,630 |
|
|
|
6,507 |
|
Operating lease right-of-use assets |
|
|
5,580 |
|
|
|
11,574 |
|
Other assets |
|
|
1,289 |
|
|
|
454 |
|
Total assets |
|
$ |
321,902 |
|
|
$ |
401,987 |
|
Liabilities and stockholders’ equity |
|
|
|
|
|
|
||
Current liabilities: |
|
|
|
|
|
|
||
Accounts payable |
|
$ |
2,608 |
|
|
$ |
2,453 |
|
Accrued compensation |
|
|
3,799 |
|
|
|
2,689 |
|
Accrued research costs |
|
|
720 |
|
|
|
633 |
|
Accrued expenses and other current liabilities |
|
|
1,871 |
|
|
|
886 |
|
Operating lease liabilities, current |
|
|
4,045 |
|
|
|
5,482 |
|
Total current liabilities |
|
|
13,043 |
|
|
|
12,143 |
|
Operating lease liabilities, non-current |
|
|
1,749 |
|
|
|
5,794 |
|
Other long- term liabilities |
|
|
10,819 |
|
|
|
— |
|
Total liabilities |
|
|
25,611 |
|
|
|
17,937 |
|
|
|
|
|
|
|
|||
Stockholders’ equity: |
|
|
|
|
|
|
||
Preferred stock, $0.00001 par value, 10,000,000 shares authorized as of December 31, 2022 and 2021; and no shares issued and outstanding as of December 31, 2022 and 2021 |
|
|
— |
|
|
|
— |
|
Common stock, $0.00001 par value, 300,000,000 shares authorized as of December 31, 2022 and December 31, 2021; 58,221,760 and 58,010,823 shares issued and outstanding as of December 31, 2022 and 2021, respectively |
|
|
1 |
|
|
|
1 |
|
Additional paid-in capital |
|
|
539,741 |
|
|
|
525,400 |
|
Accumulated other comprehensive loss |
|
|
(1,048 |
) |
|
|
— |
|
Accumulated deficit |
|
|
(242,403 |
) |
|
|
(141,351 |
) |
Total stockholders’ equity |
|
|
296,291 |
|
|
|
384,050 |
|
Total liabilities and stockholders’ equity |
|
$ |
321,902 |
|
|
$ |
401,987 |
|
The accompanying notes are an integral part of these financial statements.
117
Graphite Bio, Inc.
Statements of Operations and Comprehensive Loss
(in thousands, except share and per share data)
|
|
Year Ended |
|
|
|||||
|
|
2022 |
|
|
2021 |
|
|
||
Operating expenses: |
|
|
|
|
|
|
|
||
Research and development |
|
$ |
72,787 |
|
|
$ |
37,932 |
|
|
General and administrative |
|
|
32,852 |
|
|
|
22,511 |
|
|
Total operating expenses |
|
|
105,639 |
|
|
|
60,443 |
|
|
Loss from operations |
|
|
(105,639 |
) |
|
|
(60,443 |
) |
|
Other income (expense), net: |
|
|
|
|
|
|
|
||
Interest income, net |
|
|
4,587 |
|
|
|
24 |
|
|
Change in fair value of the Series A redeemable convertible preferred stock tranche liability |
|
|
— |
|
|
|
(10,341 |
) |
|
Total other income (expense), net |
|
|
4,587 |
|
|
|
(10,317 |
) |
|
Net loss |
|
$ |
(101,052 |
) |
|
$ |
(70,760 |
) |
|
Unrealized loss on investments in marketable securities |
|
|
(1,048 |
) |
|
|
— |
|
|
Comprehensive loss |
|
$ |
(102,100 |
) |
|
$ |
(70,760 |
) |
|
Net loss per share attributable to common stockholders—basic and diluted |
|
$ |
(1.84 |
) |
|
$ |
(2.45 |
) |
|
Weighted-average shares used in computing net loss per share—basic and diluted |
|
|
54,873,675 |
|
|
|
28,919,255 |
|
|
The accompanying notes are an integral part of these financial statements.
118
Graphite Bio, Inc.
Statements of Redeemable Convertible Preferred Stock and Stockholders’ Equity (Deficit)
(in thousands, except share data)
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Accumulated |
|
|
|
|
|
|
|
|||||||||||
|
|
Redeemable Convertible Preferred Stock |
|
|
|
Common |
|
|
Additional |
|
|
Other |
|
|
|
|
|
Total |
|
||||||||||||||||||||||||
|
|
Series A |
|
|
Series B |
|
|
|
Stock |
|
|
Paid-In |
|
|
Comprehensive |
|
|
Accumulated |
|
|
Stockholders’ |
|
|||||||||||||||||||||
|
|
Shares |
|
|
Amount |
|
|
Shares |
|
|
Amount |
|
|
|
Shares |
|
|
|
Amount |
|
|
Capital |
|
|
Loss |
|
|
Deficit |
|
|
Equity (Deficit) |
|
|||||||||||
Balance at December 31, 2020 |
|
|
30,019,945 |
|
|
$ |
55,608 |
|
|
|
— |
|
|
$ |
— |
|
|
|
|
10,279,102 |
|
|
|
$ |
— |
|
|
$ |
5,183 |
|
|
$ |
— |
|
|
$ |
(70,591 |
) |
|
$ |
(65,408 |
) |
|
Common stock issued upon exercise of options |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
33,906 |
|
|
|
|
— |
|
|
|
42 |
|
|
|
— |
|
|
|
— |
|
|
|
42 |
|
|
Common stock issued upon early exercise of options |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
918,825 |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Common stock issued under ESPP |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
39,894 |
|
|
|
|
— |
|
|
|
306 |
|
|
|
— |
|
|
|
— |
|
|
|
306 |
|
|
Issuance of Series A redeemable convertible preferred stock for cash, net of issuance costs of $4 |
|
|
15,000,000 |
|
|
|
14,997 |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Issuance of Series B redeemable convertible preferred stock for cash, net of issuance costs of $226 |
|
|
— |
|
|
|
— |
|
|
|
29,792,487 |
|
|
|
150,524 |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Reclassification of tranche liability upon settlement |
|
|
— |
|
|
|
39,403 |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Issuance of common stock in connection with License Agreement with Stanford |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
640,861 |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Repurchase of common stock in connection with terms of License Agreement with Stanford |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(624,845 |
) |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Issuance of common stock upon initial public offering, net of issuance costs |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
16,100,000 |
|
|
|
|
— |
|
|
|
251,323 |
|
|
|
— |
|
|
|
— |
|
|
|
251,323 |
|
|
Conversion of redeemable convertible preferred stock to common stock at closing of initial public offering |
|
|
(45,019,945 |
) |
|
|
(110,008 |
) |
|
|
(29,792,487 |
) |
|
|
(150,524 |
) |
|
|
|
30,761,676 |
|
|
|
|
1 |
|
|
|
260,531 |
|
|
|
— |
|
|
|
— |
|
|
|
260,532 |
|
|
Vesting of early exercised shares |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
144 |
|
|
|
— |
|
|
|
— |
|
|
|
144 |
|
|
Repurchase of unvested early exercised shares |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(138,596 |
) |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
7,871 |
|
|
|
— |
|
|
|
— |
|
|
|
7,871 |
|
|
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(70,760 |
) |
|
|
(70,760 |
) |
|
Balance at December 31, 2021 |
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
|
58,010,823 |
|
|
|
$ |
1 |
|
|
$ |
525,400 |
|
|
$ |
— |
|
|
$ |
(141,351 |
) |
|
$ |
384,050 |
|
|
Common shares issued upon exercise of options |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
67,196 |
|
|
— |
|
|
— |
|
|
|
20 |
|
|
|
— |
|
|
|
— |
|
|
|
20 |
|
Common shares issued under ESPP |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
333,155 |
|
|
— |
|
|
— |
|
|
|
656 |
|
|
|
— |
|
|
|
— |
|
|
|
656 |
|
Vesting of early exercised shares |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
|
131 |
|
|
|
— |
|
|
|
— |
|
|
|
131 |
|
Repurchase of unvested early exercised shares |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
(189,414 |
) |
|
— |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
Stock-based compensation expense |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
— |
|
|
- |
|
|
|
13,534 |
|
|
|
— |
|
|
|
— |
|
|
|
13,534 |
|
Unrealized loss on investments in marketable securities |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
|
— |
|
|
|
(1,048 |
) |
|
|
— |
|
|
|
(1,048 |
) |
Net loss |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
|
— |
|
|
— |
|
|
— |
|
|
|
— |
|
|
|
— |
|
|
|
(101,052 |
) |
|
|
(101,052 |
) |
Balance at December 31, 2022 |
|
|
— |
|
|
$ |
— |
|
|
|
— |
|
|
$ |
— |
|
|
|
|
58,221,760 |
|
|
|
$ |
1 |
|
|
$ |
539,741 |
|
|
$ |
(1,048 |
) |
|
$ |
(242,403 |
) |
|
$ |
296,291 |
|
|
The accompanying notes are an integral part of these financial statements
119
Graphite Bio, Inc.
Statements of Cash Flows
(in thousands)
|
|
Year Ended |
|
|||||||
|
|
December 31, |
|
|||||||
|
|
2022 |
|
|
|
2021 |
|
|||
Cash flows from operating activities: |
|
|
|
|
|
|
|
|
||
Net loss |
|
$ |
|
(101,052 |
) |
|
$ |
|
(70,760 |
) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
||
Net amortization of premiums and discounts on investments in marketable securities |
|
|
|
(1,600 |
) |
|
|
|
— |
|
Depreciation and amortization |
|
|
|
2,352 |
|
|
|
|
734 |
|
Noncash lease expense |
|
|
|
5,994 |
|
|
|
|
1,460 |
|
Stock-based compensation expense |
|
|
|
13,534 |
|
|
|
|
7,871 |
|
Change in fair value of the Series A redeemable convertible preferred stock tranche |
|
|
|
— |
|
|
|
|
10,341 |
|
Changes in assets and liabilities: |
|
|
|
|
|
|
|
|
||
Prepaid expenses and other current assets and other assets |
|
|
|
(3,211 |
) |
|
|
|
(3,914 |
) |
Accounts payable |
|
|
|
194 |
|
|
|
|
1,825 |
|
Accrued compensation |
|
|
|
1,110 |
|
|
|
|
2,223 |
|
Accrued research costs |
|
|
|
87 |
|
|
|
|
(1,131 |
) |
Accrued expenses and other current liabilities and other liabilities |
|
|
|
94 |
|
|
|
|
257 |
|
Operating lease liabilities |
|
|
|
(5,482 |
) |
|
|
|
(1,758 |
) |
Net cash used in operating activities |
|
|
|
(87,980 |
) |
|
|
|
(52,852 |
) |
Cash flows from investing activities: |
|
|
|
|
|
|
|
|
||
Purchases of property and equipment |
|
|
|
(6,594 |
) |
|
|
|
(5,740 |
) |
Purchases of investments in marketable securities |
|
|
|
(405,519 |
) |
|
|
|
— |
|
Proceeds from maturities of marketable securities |
|
|
|
170,250 |
|
|
|
|
— |
|
Net cash used in investing activities |
|
|
|
(241,863 |
) |
|
|
|
(5,740 |
) |
Cash flows from financing activities: |
|
|
|
|
|
|
|
|
||
Proceeds from issuance of redeemable convertible preferred stock, net of issuance costs |
|
|
|
— |
|
|
|
|
165,521 |
|
Proceeds from initial public offering, net of issuance costs |
|
|
|
— |
|
|
|
|
251,323 |
|
Proceeds from issuance of common stock upon exercise of vested stock options |
|
|
|
20 |
|
|
|
|
28 |
|
Proceeds from employee stock purchase plan |
|
|
|
656 |
|
|
|
|
306 |
|
Proceeds from issuance of common stock upon early exercises of stock options |
|
|
|
— |
|
|
|
|
289 |
|
Repurchase of unvested early exercised shares |
|
|
|
(79 |
) |
|
|
|
— |
|
Net cash provided by financing activities |
|
|
|
597 |
|
|
|
|
417,467 |
|
Net increase (decrease) in cash, cash equivalents and restricted cash |
|
|
|
(329,246 |
) |
|
|
|
358,875 |
|
Cash, cash equivalents and restricted cash, at beginning of period |
|
|
|
378,692 |
|
|
|
|
19,817 |
|
Cash, cash equivalents and restricted cash, at end of period |
|
$ |
|
49,446 |
|
|
$ |
|
378,692 |
|
Reconciliation of cash, cash equivalents and restricted cash to statement of financial position: |
|
|
|
|
|
|
|
|
||
Cash and cash equivalents |
|
|
|
47,730 |
|
|
|
|
376,976 |
|
Restricted cash |
|
|
|
1,716 |
|
|
|
|
1,716 |
|
Cash, cash equivalents and restricted cash in statement of financial position |
|
$ |
|
49,446 |
|
|
$ |
|
378,692 |
|
Supplemental disclosures of non-cash investing and financing information: |
|
|
|
|
|
|
|
|
||
Conversion of redeemable convertible preferred stock to common stock at closing of initial public offering |
|
$ |
|
— |
|
|
$ |
|
260,532 |
|
Property and equipment purchases in accounts payable and accrued expenses |
|
$ |
|
(36 |
) |
|
$ |
|
(75 |
) |
Settlement of redeemable convertible preferred stock tranche liability |
|
$ |
|
— |
|
|
$ |
|
(39,403 |
) |
Additions to right-of-use assets from new operating lease liabilities |
|
$ |
|
— |
|
|
$ |
|
13,034 |
|
Lessor funded lease incentives included in property and equipment |
|
$ |
|
11,920 |
|
|
$ |
|
— |
|
Vesting of early exercised stock options |
|
$ |
|
131 |
|
|
$ |
|
144 |
|
Repurchase of unvested early exercised shares included in accounts payable |
|
$ |
|
(17 |
) |
|
$ |
|
(42 |
) |
Proceeds from issuance of common stock upon exercise of stock options included in accounts receivable |
|
$ |
|
|
|
$ |
|
(14 |
) |
|
The accompanying notes are an integral part of these financial statements.
120
Graphite Bio, Inc.
Notes to Financial Statements
1. Description of Business, Organization and Liquidity
Organization and Business
Graphite Bio, Inc. (the “Company”) is a clinical-stage, next-generation gene editing company harnessing high-efficiency targeted gene integration to develop a new class of therapies to potentially cure a wide range of serious and life-threatening diseases. The Company is pioneering a precision gene editing approach to achieve one of medicine’s most elusive goals: to precisely “find & replace” any gene in the genome. The Company’s next-generation gene editing platform was designed to allow the Company to precisely correct mutations, replace entire disease-causing genes with normal genes, or insert new genes into predetermined, safe locations. In January 2023, the Company announced a voluntary pause of our Phase 1/2 CEDAR study of nulabeglogene autogedtemcel (nula-cel, formerly GPH101), its lead product candidate for sickle cell disease (SCD), due to a serious adverse event in the first patient dosed, which the Company concluded is likely related to study treatment. Nula-cel is a highly differentiated approach with the potential to directly correct the mutation that causes SCD and restore normal adult hemoglobin (HgbA) expression. In February 2023, the Company announced its decision to discontinue the development of nula-cel and initiate a process to explore strategic alternatives. From its inception in 2017, the Company’s primary activities have been to perform research and development, undertake preclinical studies and enable manufacturing activities in support of its product development efforts, organize and staff the Company, establish its intellectual property portfolio, and raise capital to support and expand such activities.
The Company was incorporated in Ontario, Canada in June 2017 as Longbow Therapeutics Inc., and was reincorporated in the State of Delaware in October 2019. In February 2020, the Company changed its name to Integral Medicines, Inc., and again in August 2020, changed the name to Graphite Bio, Inc. Research and development of the Company’s initial technology ceased at the end of 2018, and the Company did not have any significant operations or any research and development activities in 2019. In March 2020, the Company identified new gene editing technology which the Company sought to further develop, and the Company licensed the related intellectual property rights from The Board of Trustees of the Leland Stanford Junior University (“Stanford”) in December 2020 (Note 6).
Reverse Stock Split
On June 18, 2021, the Company’s board of directors approved an amendment to the Company’s amended and restated certificate of incorporation to effect a reverse stock split of the Company’s issued and outstanding common stock at a 1 for 2.432 ratio, which was effected on June 21, 2021. The par value and authorized shares of common stock and convertible preferred stock were not adjusted as a result of the reverse stock split. All issued and outstanding common stock, options to purchase common stock and per share amounts contained in the financial statements have been retroactively adjusted to reflect the reverse stock split for all periods presented. The financial statements have also been retroactively adjusted to reflect a proportional adjustment to the conversion ratio for each series of preferred stock that was effected in connection with the reverse stock split.
Initial Public Offering
On June 24, 2021, the Company’s registration statement on Form S-1 relating to its initial public offering (“IPO”) was declared effective by the Securities and Exchange Commission (“SEC”) and the shares of its common stock began trading on the Nasdaq Global Market on June 25, 2021. The IPO closed on June 29, 2021, pursuant to which the Company issued and sold 14,000,000 shares of its common stock at a public offering price of $17.00 per share. On July 2, 2021, the Company issued 2,100,000 shares of its common stock to the underwriters of the IPO pursuant to the full exercise of their option to purchase additional shares. The Company received in June and July 2021 net proceeds of approximately $251.3 million from the IPO, after deducting underwriting discounts and commissions of $19.1 million and offering costs of approximately $3.2 million. Prior to the completion of the IPO, all shares of redeemable convertible preferred stock then outstanding were converted into 30,761,676 shares of common stock.
Liquidity Matters
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. The Company’s operations have historically been financed through the issuance of common and redeemable convertible preferred stock. The Company has incurred significant operating losses and negative net cash flows from operations since inception. During the year ended December 31, 2022, the Company incurred a net loss of $101.1 million and had negative net cash flows from operating activities of $88 million. The Company had an accumulated deficit of $242.4 million as of December 31, 2022 and will continue to require substantial additional capital for research and development activities. The Company anticipates incurring additional losses until such time, if ever, that it can generate significant sales of its drug candidates currently in development. As of December 31, 2022, the Company had cash and cash equivalents and investments in marketable securities of $283.6 million.
On July 21, 2022, the Company filed a shelf registration statement on Form S-3 (the “2022 Shelf”) with the SEC in relation to the registration of up to an aggregate offering price of $300.0 million of common stock, preferred stock, debt securities, warrants and units or any combination thereof. The Company also simultaneously entered into a Controlled Equity OfferingSM Sales Agreement with
121
Cantor Fitzgerald & Co. (the “Sales Agent”), to provide for the offering, issuance and sale by the Company of up to an aggregate of $75.0 million of its common stock from time to time in “at-the-market” offerings under the 2022 Shelf and subject to the limitations thereof (the “Sales Agreement”). The Company will pay to the Sales Agent cash commissions of up to 3.0 percent of the gross proceeds of sales of common stock under the Sales Agreement. The Company has not issued any shares or received any proceeds from any offerings under the 2022 Shelf through March 15, 2023.
Management believes that its existing cash, cash equivalents and investments in marketable securities are sufficient to continue operating activities for at least 12 months following the issuance date of these financial statements.
2. Summary of Significant Accounting Policies
Basis of Presentation
These financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America (“U.S. GAAP”).
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of expenses during the reporting period. On an ongoing basis, the Company evaluates estimates and assumptions, including but not limited to those related to the fair value of the marketable securities, the fair value of redeemable convertible preferred stock and common stock, stock-based compensation expense, accruals for research and development costs, lease assets and liabilities, the valuation of deferred tax assets, and uncertain income tax positions. Management bases its estimates on historical experience and on various other assumptions that are believed to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities that are not readily apparent from other sources. Actual results may differ materially from those estimates.
Concentration of Credit Risk
Cash and cash equivalents are financial instruments that potentially subject the Company to concentrations of credit risk. Substantially all of the Company’s cash and cash equivalents are deposited in accounts with major financial institution and amounts may exceed federally insured limits. Management believes that the Company is not exposed to significant credit risk due to the financial strength of the depository institution in which the cash and cash equivalents are held. The Company has not experienced any losses on deposits of cash and cash equivalents.
Risks and Uncertainties
The Company is subject to certain risks and uncertainties, including, but not limited to, changes in any of the following areas that the Company believes could have a material adverse effect on the future financial position or results of operations: the timing of, and the Company’s ability to advance its current and future product candidates into and through clinical development; costs and timelines associated with the manufacture of clinical supplies of the Company’s product candidates; regulatory approval and market acceptance of, and reimbursement for its product candidates; performance of third-party CROs and CMOs; competition from pharmaceutical companies with greater financial resources or expertise; protection of the intellectual property; litigation or claims against the Company based on intellectual property or other factors; and its ability to attract and retain employees necessary to support its growth. Disruption from CROs’, CMOs’ or suppliers’ operations would likely have a negative impact on the Company’s business, financial position and results of operations.
Segment and Geographical Information
The Company operates and manages its business as one reportable and operating segment. The chief executive officer, who is the chief operating decision maker, reviews financial information on an aggregate basis for purposes of allocating resources and evaluating financial performance. All of the Company’s long-lived assets are based in the United States.
Cash and Cash Equivalents
The Company considers all highly liquid investments purchased with a maturity of three months or less at the date of purchase to be cash equivalents. As of December 31, 2022 and 2021, cash and cash equivalents consisted of cash, money market funds, and commercial paper.
Restricted Cash
Restricted cash of $1.7 million as of December 31, 2022 and 2021 represented security deposits in the form of letters of credit issued in connection with the leases of the Company’s headquarters (Notes 6 and 8).
122
Marketable Securities
The Company’s marketable securities are accounted for as available-for-sale and recorded at fair value with the related unrealized gains and losses included in accumulated other comprehensive gain (loss).
The Company reviews its investment portfolio to identify and evaluate investments that have an indication of possible other-than-temporary impairment. Factors considered in determining whether a loss is other-than-temporary include the length of time and extent to which fair value has been less than the cost basis, the financial condition and near-term prospects of the investee and the Company’s intent and ability to hold the investment for a period of time sufficient to allow for any anticipated recovery in market value.
Fair Value Measurements
Fair value is defined as the price that would be received to sell an asset or paid to transfer a liability in an orderly transaction between market participants at the measurement date. As such, fair value is a market-based measurement that should be determined based on assumptions that market participants would use in pricing an asset or liability. The carrying amounts of financial instruments, including restricted cash, prepaid expenses and other current assets, accounts payable, accrued compensation, accrued expenses, and other liabilities, approximate fair value due to their short-term maturities. The cash invested in money-market funds and redeemable convertible preferred stock tranche liability are carried at fair value.
Property and Equipment, Net
Property and equipment are recorded at cost, less accumulated depreciation and amortization. Depreciation is computed using the straight-line method over the estimated useful lives of the assets, generally to five years. Repairs and maintenance expenditures, which are not considered improvements and do not extend the useful life of property and equipment, are expensed as incurred. When assets are retired or otherwise disposed of, the cost and related accumulated depreciation and amortization are removed from the balance sheet and the resulting gain or loss is reflected in the statements of operations and comprehensive loss in the period realized.
Leases
Effective January 1, 2021, the Company adopted ASC Topic 842, Leases (“ASC 842”) using the optional transition method and applied the standard only to leases that existed at that date. Under the optional transition method, the Company does not need to restate the comparative periods in transition and will continue to present financial information and disclosures for periods before January 1, 2021 in accordance with ASC Topic 840. The Company has elected the package of practical expedients allowed under ASC Topic 842, which permits the Company to account for its existing operating leases as operating leases under the new guidance, without reassessing the Company’s prior conclusions about lease identification, lease classification and initial direct cost. As a result of the adoption of the new lease accounting guidance on January 1, 2021, the Company recognized no cumulative adjustment to accumulated deficit since the Company had only one operating lease with a term of less than 12 months and no plans to extend the lease.
On January 27, 2021, the Company entered into a lease agreement for office and lab space in South San Francisco, CA that included two office suites. The lease terms for the office suites commenced in July and August 2021, respectively. Upon commencement of the leases, the Company recognized operating lease right-of-use assets of $4.1 million and operating lease liabilities of $4.1 million (see Note 8).
On August 30, 2021, the Company entered into a service agreement with The Laboratory for Cell and Gene Medicine (“LCGM”) that included an embedded operating lease as the manufacturing suite in LCGM’s facility is designated for the Company’s exclusive use though April 30, 2023. Upon commencement of the agreement, the Company recognized operating lease right-of-use assets of $5.3 million and operating lease liabilities of $5.3 million (see Notes 6 and 8).
On November 10, 2021, the Company entered into a sublease agreement for office space in Brisbane, CA. The lease terms for the sublease commenced in December 2021. Upon commencement of the lease, the Company recognized operating lease right-of-use assets of $3.2 million and operating lease liabilities of $3.2 million (see Note 8).
On November 17, 2021, the Company entered into a service agreement with Explora BioLabs, Inc (“Explora”) that included an embedded operating lease as the vivarium space is designated for the Company’s exclusive use through November 2023. Upon commencement of the lease, the Company recognized operating lease right-of-use assets of $0.6 million and operating lease liabilities of $0.6 million (see Note 8).
The Company determines the initial classification and measurement of its right-of-use assets and lease liabilities at the lease commencement date and thereafter if modified. The lease term includes any renewal options and termination options that the Company is reasonably assured to exercise. The present value of lease payments is determined by using the interest rate implicit in the lease, if that rate is readily determinable; otherwise, the Company uses its incremental borrowing rate. The incremental borrowing rate is determined by using the rate of interest that the Company would pay to borrow on a collateralized basis an amount equal to the lease payments for a similar term and in a similar economic environment.
123
Fixed lease expense for operating leases is recognized on a straight-line basis, unless the right-of-use assets have been impaired, over the reasonably assured lease term based on the total lease payments and is included in operating expenses in the statements of operations and comprehensive loss. Variable lease expenses are recognized as incurred. Fixed and variable lease expense on operating leases is recognized within operating expenses in the statements of operations and comprehensive loss.
For operating leases for which the right-of-use assets have been impaired, the Company will recognize the amortization of the right-of-use assets on a straight-line basis over the remaining respective lease term with lease expense included in operating expenses in the statements of operations and comprehensive loss.
For all leases, rent payments that are based on a fixed index or rate at the lease commencement date are included in the measurement of lease assets and lease liabilities at the lease commencement date.
The Company has elected the practical expedient to not separate lease and non-lease components. The Company’s non-lease components are primarily related to maintenance, insurance and taxes, which varies based on future outcomes and is thus recognized in lease expense when incurred.
Asset Acquisitions
The Company measures and recognizes asset acquisitions that are not deemed to be business combinations based on the cost to acquire the assets, which includes transaction costs. Goodwill is not recognized in asset acquisitions. In an asset acquisition, the cost allocated to acquire in-process research and development (“IPR&D”) with no alternative future use is charged to research and development expense at the acquisition date. Please refer to Note 6 for more details on asset acquisition.
Impairment of Long-Lived Assets
The Company reviews long-lived assets for impairment whenever events or changes in circumstances indicate that the carrying amount of an asset may not be recoverable. Recoverability is measured by comparing the carrying amount to the future undiscounted net cash flows which the assets are expected to generate. If such assets are considered to be impaired, the impairment to be recognized is measured as the amount by which the carrying amount of the assets exceeds the projected discounted future net cash flows generated by the assets. There have been no such impairments of long-lived assets in the years ended December 31, 2022 and 2021.
Research and Development Expenses
Research and development costs are expensed as incurred. Research and development costs include salaries, stock-based compensation, and benefits for employees performing research and development activities, an allocation of facility and overhead expenses, expenses incurred under agreements with consultants, CMOs, CROs and investigative sites that conduct preclinical studies, other supplies and costs associated with product development efforts, preclinical activities, and regulatory operations.
Accrued Research and Development Expenses
The Company has entered into various agreements with outsourced vendors, CROs and CMOs. Research and development accruals are estimated based on the level of services performed, progress of the studies, including the phase or completion of events, and contracted costs. The estimated costs of research and development services provided, but not yet invoiced, are included in accrued research costs on the balance sheets. If the actual timing of the performance of services or the level of effort varies from the original estimates, the Company will adjust the accrual accordingly. Payments made under these arrangements in advance of the performance of the related services are recorded as prepaid expenses and other current assets until the services are rendered. To date, there have been no material differences between estimates of such expenses and the amounts actually incurred.
Tax Credit Receivable
The Company is eligible for federal and California research and development credits for its research and development activities performed within the United States and California, respectively. The credits are, generally, available to offset federal and California income tax liabilities as applicable. The Company has applied $0.1 million of federal research and development credits to offset its federal payroll tax expenses as of the year ended December 31, 2022 due to its small business status. The Company is electing to utilize $0.3 million of current year R&D credit generated against the employer portion of the payroll tax.
Income Taxes
The Company accounts for income taxes using the asset and liability method. The Company recognizes deferred tax assets and liabilities for the expected future tax consequences of events that have been included in the financial statements or tax returns. Deferred tax assets and liabilities are determined based on the difference between the financial statements and tax basis of assets and liabilities using enacted tax rates in effect for the year in which the differences are expected to reverse.
In evaluating the ability to recover deferred income tax assets, the Company considers all available positive and negative evidence, including operating results, ongoing tax planning and forecasts of future taxable income on a jurisdiction-by-jurisdiction basis. In the event the Company determines that it would be able to realize deferred income tax assets in the future in excess of their net recorded amount, the Company would make an adjustment to the valuation allowance that would reduce the provision for income taxes.
124
Conversely, in the event that all or part of the net deferred tax assets are determined not to be realizable in the future, an adjustment to the valuation allowance would be charged to earnings in the period when such determination is made. As of December 31, 2022 and 2021, the Company has recorded a full valuation allowance on deferred tax assets.
Tax benefits related to uncertain tax positions are recognized when it is more likely than not that a tax position will be sustained during an audit. Interest and penalties related to unrecognized tax benefits are included within the provision for income tax.
Stock-Based Compensation Expense
The Company’s stock-based equity awards include restricted stock awards and stock options that are granted to employees and consultants that are accounted at fair value on the award grant date. Stock-based compensation expense is recognized over the awards’ vesting period on a straight-line basis and recorded as either research and development or general and administrative expenses in the statements of operations and comprehensive loss based on the function to which the related services are provided. Forfeitures are accounted for as they occur.
As there was no public market for the Company’s common stock prior to the initial public offering of its common stock in June 2021, the estimated fair value of common stock was determined by the Company’s board of directors as of the date of each option grant, with input from management, considering third-party valuations of its common stock, as well as the Company’s board of directors’ assessment of additional objective and subjective factors that it believed were relevant, and which may have changed from the date of the most recent third-party valuation through the date of the grant. These third-party valuations were performed in accordance with the guidance outlined in the American Institute of Certified Public Accountants’ Accounting and Valuation Guide, Valuation of Privately Held Company Equity Securities Issued as Compensation. Following the closing of the initial public offering, the fair value of the Company’s common stock is determined based on the quoted market price of common stock. The Company also lacks company‑specific historical and implied volatility information for its stock. The Company estimates its expected stock price volatility and expected term based on the historical volatility and expected term of publicly traded peer companies and expects to continue to do so until such time as it has adequate historical data regarding the volatility of its own traded stock price. The risk‑free interest rate is determined by reference to the U.S. Treasury yield curve in effect at the time of grant of the award for time periods approximately equal to the expected term of the award. There is no expected dividend yield since the Company has never paid cash dividends on common stock and does not expect to pay any cash dividends in the foreseeable future.
Employee Stock Purchase Plan
The Company recognizes stock-based expense related to shares issued pursuant to its Employee Stock Purchase Plan on a straight-line basis over the offering period, which is typically 6 months. The first offering period commenced on June 25, 2021 and ended on November 30, 2021. The second, third, and fourth offering periods commenced on December 1, 2021, June 1, 2022, and December 1, 2022, respectively. The ESPP allows eligible employees to purchase shares of the Company's common stock at a 15 percent discount on the lower price of either (i) the offering period begin date or (ii) the purchase date. The Company estimates the fair value of shares to be issued under the ESPP using the Black-Scholes option-pricing model.
Comprehensive Loss
Comprehensive loss is defined as the change in equity of a business during a period from transactions and other events and circumstances from non-owner sources. Comprehensive loss includes net loss as well as other changes in stockholders’ deficit which includes certain changes in equity that are excluded from net loss. The Company’s only element of other comprehensive loss is unrealized gains and losses on marketable securities.
Net Loss Per Share
Basic net loss per common share is calculated by dividing the net loss attributable to common stockholders by the weighted-average number of common stock outstanding during the period, without consideration of potentially dilutive securities. Diluted net loss per share is computed by dividing the net loss attributable to common stockholders by the weighted-average number of common stock and potentially dilutive securities outstanding for the period. For purposes of the diluted net loss per share calculation, redeemable convertible preferred stock, common stock subject to repurchase, restricted common shares issued, and stock options are considered to be potentially dilutive securities.
Basic and diluted net loss attributable to common stockholders per share is presented in conformity with the two-class method required for participating securities. Restricted shares issued to the founders and upon early exercise of stock options also participate in dividends from the issuance date and are considered participating securities. Participating securities do not have a contractual obligation to share in losses. As such, the net loss was attributed entirely to common stockholders. Because the Company has reported a net loss for all periods presented, diluted net loss per common share is the same as basic net loss per common share for those periods.
Adopted and Recent Accounting Pronouncements
The Company is a smaller reporting company and an emerging growth company, as defined in the Jumpstart Our Business Startups Act of 2012 (the “JOBS Act”). Under the JOBS Act, emerging growth companies can delay the adoption of new or revised accounting
125
standards issued subsequent to the enactment of the JOBS Act until such time as those standards apply to private companies. Thus, the Company has elected to use the extended transition period for complying with new or revised accounting standards that have different effective dates for public and private companies until the earlier of the date that (i) the Company is no longer an emerging growth company or (ii) the Company affirmatively and irrevocably opts out of the extended transition period provided in the JOBS Act. However as described below, the Company early adopted certain accounting standards, as the JOBS Act does not preclude an emerging growth company from adopting a new or revised accounting standard earlier than the time that such standard applies to private companies to the extent early adoption is permitted.
Recently Adopted Accounting Pronouncements
In June 2016, the FASB issued ASU 2016-13, Credit Losses. The FASB also issued amendments and the initial ASU, and all updates are included herein as the Credit Losses standard or Topic 326. The new standard updates the guidance for measuring and recording credit losses on financial assets measured at amortized cost by replacing the “incurred loss” model with an “expected loss” model. As a result of adoption, the Company would present these financial assets, which includes available-for-sale debt securities, at the net amount it expects to collect. The amendment also requires that the Company records credit losses related to its available-for-sale debt securities as an allowance through net income rather than reducing the carrying amount under the historical, other-than-temporary-impairment model. With certain exceptions, the guidance is applied using a modified retrospective approach by reflecting adjustments through a cumulative-effect impact to retained earnings as of the beginning of the fiscal year of adoption. The Company early adopted the new standard under the modified retrospective approach as of January 1, 2022 with no material impact on its financial statements.
In August 2020, the FASB issued ASU No. 2020-06, Debt—Debt with Conversion and Other Options (Subtopic 470-20) and Derivatives and Hedging—Contracts in Entity’s Own Equity (Subtopic 815-40): Accounting for Convertible Instruments and Contracts in an Entity’s Own Equity (ASU 2020-06), which simplifies the accounting for convertible instruments by reducing the number of accounting models available for convertible debt instruments. This guidance also eliminates the treasury stock method to calculate diluted earnings per share for convertible instruments and requires the use of the if-converted method. The Company adopted this standard as of January 1, 2022 utilizing the modified retrospective method, with no material impact on its financial statements. The Company does not have any convertible instruments as of December 31, 2022.
3. Fair Value Measurements
Assets and liabilities recorded at fair value on a recurring basis in the balance sheets, as well as assets and liabilities measured at fair value on a non-recurring basis or disclosed at fair value, are categorized based upon the level of judgment associated with inputs used to measure their fair values. The accounting guidance for fair value provides a framework for measuring fair value and requires certain disclosures about how fair value is determined. Fair value is defined as the price that would be received upon the sale of an asset or paid to transfer a liability (an exit price) in an orderly transaction between market participants at the reporting date.
The accounting guidance also establishes a three-level valuation hierarchy that prioritizes the inputs to valuation techniques used to measure fair value based upon whether such inputs are observable or unobservable. Observable inputs reflect market data obtained from independent sources, while unobservable inputs reflect market assumptions made by the reporting entity. The three-level hierarchy for the inputs to valuation techniques is briefly summarized as follows:
Level 1 — Inputs are unadjusted, quoted prices in active markets for identical assets or liabilities at the measurement date;
Level 2 — Inputs are observable, unadjusted quoted prices in active markets for similar assets or liabilities, unadjusted quoted prices for identical or similar assets or liabilities in markets that are not active, or other inputs that are observable or can be corroborated by observable market data for substantially the full term of the related assets or liabilities; and
Level 3 — Unobservable inputs that are significant to the measurement of the fair value of the assets or liabilities that are supported by little or no market data.
Assets and liabilities measured at fair value are classified in their entirety based on the lowest level of input that is significant to the fair value measurement. An assessment of the significance of a particular input to the fair value measurement in its entirety requires management to make judgments and consider factors specific to the asset or liability. Changes in the ability to observe valuation inputs may result in a reclassification of levels of certain securities within the fair value hierarchy. The Company recognizes transfers into and out of levels within the fair value hierarchy in the period in which the actual event or change in circumstances that caused the transfer occurs.
As of December 31, 2022 and 2021, Level 1 securities consist of U.S. Treasury and money market funds, for which the carrying amounts are based on the quoted market prices in active markets
As of December 31, 2022, Level 2 securities consist of highly rated commercial paper, U.S. agency securities, and asset-backed securities, for which fair value is determined through the use of models or other valuation methodologies. As of December 31, 2022, the Company had an immaterial amount of unrealized gains on its Level 2 securities.
126
During the periods presented, the Company did not have any Level 3 securities.
The following tables set forth the financial instruments that were measured at fair value on a recurring basis by level within the fair value hierarchy as of December 31, 2022 and 2021 (in thousands):
|
|
December 31, 2022 |
|
|||||||||||||
|
|
Total Fair |
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
||||
Cash equivalents: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
Money market funds (1) |
|
$ |
45,739 |
|
|
$ |
45,739 |
|
|
$ |
|
|
$ |
|
||
Commercial paper (1) |
|
|
1,991 |
|
|
|
|
|
|
1,991 |
|
|
|
|
||
Total cash equivalents |
|
|
47,730 |
|
|
|
45,739 |
|
|
|
1,991 |
|
|
|
|
|
Marketable securities: |
|
|
|
|
|
|
|
|
|
|
|
|
||||
U.S. treasuries |
|
|
65,391 |
|
|
|
65,391 |
|
|
|
|
|
|
|
||
Commercial paper |
|
|
115,061 |
|
|
|
|
|
|
115,061 |
|
|
|
|
||
U.S. agency securities(2) |
|
|
53,455 |
|
|
|
|
|
|
53,455 |
|
|
|
|
||
Asset-backed securities(2) |
|
|
1,914 |
|
|
|
|
|
|
1,914 |
|
|
|
|
||
Total marketable securities |
|
|
235,821 |
|
|
|
65,391 |
|
|
|
170,430 |
|
|
|
|
|
Total cash equivalents and marketable securities |
|
$ |
283,551 |
|
|
$ |
111,130 |
|
|
$ |
172,421 |
|
|
$ |
|
|
|
|
December 31, 2021 |
|
|||||||||||||
|
|
Total Fair |
|
|
Level 1 |
|
|
Level 2 |
|
|
Level 3 |
|
||||
Money market funds (1) |
|
$ |
376,976 |
|
|
$ |
376,976 |
|
|
$ |
|
|
$ |
|
||
4. Marketable Securities
All marketable securities were considered available-for-sale as of December 31, 2022. The amortized cost, gross unrealized holding gains or losses, and fair value of the Company’s marketable securities by major security type are summarized in the table below (in thousands):
|
|
December 31, 2022 |
|
|||||||||||||
|
|
Amortized |
|
|
Unrealized |
|
|
Unrealized |
|
|
Fair |
|
||||
Available-for-sale securities |
|
|
|
|
|
|
|
|
|
|
|
|
||||
U.S. treasuries |
|
$ |
65,807 |
|
|
$ |
— |
|
|
$ |
(416 |
) |
|
$ |
65,391 |
|
Commercial paper |
|
|
115,381 |
|
|
|
13 |
|
|
|
(333 |
) |
|
|
115,061 |
|
U.S. agency securities |
|
|
53,767 |
|
|
|
15 |
|
|
|
(327 |
) |
|
|
53,455 |
|
Asset-backed securities |
|
|
1,914 |
|
|
|
— |
|
|
|
— |
|
|
|
1,914 |
|
Total available-for-sale securities |
|
$ |
236,869 |
|
|
$ |
28 |
|
|
$ |
(1,076 |
) |
|
$ |
235,821 |
|
The amortized cost of available-for-sale securities is adjusted for amortization of premiums and accretion of discounts to maturity. As of December 31, 2022, the aggregate fair value of securities with remaining maturities of less than one year held by the Company in an unrealized loss position was $220.5 million. The aggregate fair value of securities with remaining maturities of more than one year held by the Company in an unrealized loss position was $15.3 million. The Company has the intent and ability to hold such securities until recovery and has determined that there has been no material change to its credit risk. As a result, the Company determined it did not hold any investments with a credit loss at December 31, 2022.
There were no realized gains or losses recognized on the sale or maturity of available-for-sale securities during year ended December 31, 2021, and as a result, there were no reclassifications out of accumulated other comprehensive gain (loss) for the same periods.
The Company did not hold any marketable securities as of December 31, 2021.
127
5. Balance Sheet Components
Prepaid Expenses and Other Current Assets
Prepaid expenses and other current assets as of December 31, 2022 and 2021 consisted of the following (in thousands):
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
||
Advances to suppliers |
|
$ |
2,486 |
|
|
$ |
1,834 |
|
Prepaid insurance |
|
|
1,343 |
|
|
|
1,543 |
|
Other prepaid expenses |
|
|
3,307 |
|
|
|
1,383 |
|
Total prepaid expenses and other current assets |
|
$ |
7,136 |
|
|
$ |
4,760 |
|
Property and Equipment, Net
Property and equipment, net as of December 31, 2022 and 2021 consisted of the following (in thousands):
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
||
Furniture and fixtures |
|
$ |
321 |
|
|
$ |
3 |
|
Computers and network equipment |
|
|
251 |
|
|
|
108 |
|
Lab equipment |
|
|
12,521 |
|
|
|
6,680 |
|
Leasehold improvements |
|
|
304 |
|
|
|
94 |
|
Construction-in-progress |
|
|
12,440 |
|
|
|
477 |
|
Total property and equipment |
|
|
25,837 |
|
|
|
7,362 |
|
Less: accumulated depreciation |
|
|
(3,207 |
) |
|
|
(855 |
) |
Total property and equipment, net |
|
$ |
22,630 |
|
|
$ |
6,507 |
|
Depreciation expense for the years ended December 31, 2022 and 2021 was $2.4 and $0.7 million, respectively.
Accrued Expenses
Accrued expenses as of December 31, 2022 and 2021 consisted of the following (in thousands):
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
||
Professional fees |
|
$ |
367 |
|
|
$ |
186 |
|
Early exercise liability |
|
|
150 |
|
|
|
337 |
|
Other accrued expenses |
|
|
1,354 |
|
|
|
363 |
|
Total accrued expenses and other current liabilities |
|
$ |
1,871 |
|
|
$ |
886 |
|
128
6. Significant Agreements
Stanford Exclusive License Agreement
In December 2020, the Company entered into an exclusive license agreement (the “License Agreement”), with The Board of Trustees of the Leland Stanford Junior University (Stanford), pursuant to which Stanford granted us a worldwide license to specified technology and patent rights to develop, manufacture and commercialize human prophylactic and therapeutic products. Other than with respect to specified, broadly applicable assays and procedures and subject to retained rights by Stanford, the license is exclusive with respect to human prophylactic and therapeutic products for the treatment of SCD, XSCID and beta thalassemia. The license is non-exclusive with respect to those broadly applicable assays and procedures and with respect to all human prophylactic and therapeutic products other than for the treatment of SCD, XSCID and beta thalassemia.
Pursuant to the License Agreement, the Company paid an upfront license fee of $50.0 thousand, and as additional consideration for the license, the Company agreed to issue to Stanford approximately 640,861 shares of our common stock. As of December 31, 2020, the Company recorded its obligations to issue Stanford shares of common stock at an estimated fair value of $2.8 million to additional paid in capital. The common shares are expected to be issued when Stanford provides the inventors’ names for allocation of the shares. Stanford also had an option to buy up to 10% of newly issued shares in the future private financings at the price paid by other participating investors. During the year ended December 31, 2021, the Company entered into amendments to the License Agreement, pursuant to which it extended the time when the shares will be issued to May 7, 2021.
On May 7, 2021, the Company issued an aggregate of 640,861 shares of the Company’s common stock to Stanford and certain individuals designated by Stanford in consideration for rights granted to the Company under the Company’s exclusive license agreement.
On June 18, 2021, the Company exercised its right to repurchase an aggregate of 624,845 shares from each founder and investor under the Stanford Adjustment Repurchase Right as described below.
The acquisition of the exclusive license, including patent rights and know-how, and clinical supplies was accounted for as an asset acquisition and as the acquired technology and inventories did not have an alternative use, the total consideration of $2.8 million was recorded as research and development expense in the statements of operations and comprehensive loss for the year ended December 31, 2020.
In connection with the License Agreement, the Company reimbursed Stanford $0.2 million for previously incurred patent costs, which were recorded in general and administrative expenses for the year ended December 31, 2020 and, in addition, is obligated to reimburse future patent costs. During the year ended December 31, 2021, the reimbursements of patent costs to Stanford were minimal. During the year ended December 31, 2021, the Company has recognized a minimal amount in research and development expense in connection with the License Agreement
The Company is also obligated to pay annual maintenance fees as follows: $5.0 thousand in the first year, $10.0 thousand in each year 2 and 3, $25.0 thousand in each year 3 through 6, $50.0 thousand each subsequent year until first commercial sale and $200.0 thousand each subsequent year after the first commercial sale.
The Company is also obligated to make future development and regulatory milestone payments in total of up to $5.3 million, sales-based milestone payments of up to $7.5 million and royalties on future sales at percentage rates ranging in the low single digits. In addition, if the Company receives any sublicense income, it is required to share it with Stanford as a certain percentage defined for each milestone in the License Agreement. The Company will record the maintenance fees, when payable, and will record milestones when contingencies are resolved, and milestones are due. No milestones were achieved and recorded as of December 31, 2022 and 2021.
The term of the License Agreement expires on the later of (a) the expiration of the last patent or abandonment of the last patent application within the license patent rights or (b) the expiration of all royalty terms with respect to Licensed Products.
The Stanford License terminates on a product by product and country by country basis on the latest to occur of (i) expiration of the last valid claim of a licensed patent that covers the sale or manufacture of the applicable licensed product in such country, (ii) expiration of any period of regulatory exclusivity granted with respect to such licensed product in such country or (iii) ten years after the first commercial sale of such licensed product in a country Stanford also has a right to terminate the agreement if milestones plan is rejected by Stanford as specified in the License Agreement.
In January 2021, the Company entered into an option agreement (the “First Option Agreement”), with Stanford, pursuant to which Stanford granted the Company the right to obtain a license to specified patent rights relating to human prophylactic and therapeutic products. The Company may exercise the option in whole or in part to obtain a license under one or more of the optioned patent rights.
Subject to the Company’s exercise of the option under the First Option Agreement and its execution of an amendment to the License Agreement that incorporates the optioned patent rights and any optioned technology, the Company has agreed to issue to Stanford 132,137 shares of its common stock and pay a license execution fee of $10.0 thousand.
The term of the First Option Agreement expires 18 months after its effective date, subject to the Company’s right to extend such expiration date by up to an additional one year upon notice to Stanford and by another additional one year upon the reasonable agreement
129
of Stanford. The First Option Agreement will terminate if the License Agreement terminates. On June 23, 2022, the Company exercised its right to extend the term of the First Option Agreement for an additional year. As of December 31, 2022, the Company had not exercised the option under the First Option Agreement and no fees have been paid for the First Option Agreement.
In April 2021, the Company entered into an option agreement (the “Second Option Agreement”) with Stanford to negotiate the license for additional technologies from Stanford. Pursuant to the Second Option Agreement, the Company agreed to pay Stanford option fees in an aggregate amount of $30.0 thousand over the term of the option. On April 13, 2022, the Company entered into an amendment to the Second Option Agreement which extended the term for an additional year and the maintenance fee of $10.0 thousand for the extension was paid in the year ended December 31, 2022. As of December 31, 2022, the Company had not exercised the option under the Second Option Agreement.
LCGM Service Agreement
On August 30, 2021, the Company entered into a Master Manufacturing and Service Agreement with the Laboratory for Cell & Gene Medicine at Stanford (“LCGM MSA”). Pursuant to the LCGM MSA, LCGM will conduct clinical manufacturing, release testing, and product release for nula-cel in the Company’s Phase 1/2 CEDAR clinical trial to treat SCD. During 2021, the Company entered into various SOWs under the LCGM MSA under which it received technology transfer and related services for HBB Beta-Globin Gene Variant for SCD, manufacturing engineer test runs, the exclusive use of a manufacturing suite at the LCGM facility, and Phase 1/2 CEDAR clinical development and manufacturing of the HBB Variant for SCD. During the years ended December 31, 2022 and 2021, the Company has recognized $6.1 and $2.7 million in research and development expense in connection with the LCGM MSA.
IDT License Agreement
On June 7, 2021, the Company entered into a License Agreement (“IDT License Agreement”) with Integrated DNA Technologies, Inc. (“IDT”). Pursuant to the IDT License Agreement, IDT granted the Company and its affiliates a worldwide, non-exclusive, sublicensable license to research and develop products incorporating HiFi Cas9 protein variants for use in human therapeutic applications for SCD, XSCID and Gaucher disease (the Field) and a worldwide, exclusive, sublicensable license to commercialize such products in the Field. The Company has also been granted the right to expand the licensed Field to include human therapeutic applications in the additional fields of beta thalassemia disorder and lysosomal storage disorders upon the payment of an exercise fee in the amount of $0.5 million per additional field or $1.0 million for both additional fields.
In consideration of the licenses and rights granted to the Company under the IDT License Agreement, the Company agreed to pay to IDT an upfront payment in the amount of $3.0 million and up to $5.3 million (or $8.8 million if the Company elects to expand the Field as described above to include both the beta thalassemia and lysosomal storage disorders fields) in total regulatory milestone payments. Each regulatory milestone payment is payable once on an indication-by-indication basis. In addition, the Company has agreed to pay IDT a low single-digit royalty on the net sales of products, subject to reductions in specified circumstances. The acquisition of the license was accounted for as an asset acquisition and as the acquired technology did not have an alternative use, the total consideration of $3.0 million was recorded as research and development expense in the statement of operations and comprehensive loss for the year ended December 31, 2021.
The IDT License Agreement remains in effect on a country-by-country and product-by-product basis until the expiration of the royalty term for such product in such jurisdiction. The Company and IDT each have the right to terminate the IDT License Agreement for the other party’s material breach of its obligations under the IDT License Agreement, subject to specified rights to cure. Additionally, the Company may terminate the IDT License Agreement for any reason upon written notice.
During the years ended December 31, 2022 and 2021, the Company has recognized $0.0 and $3.0 million, respectively, in research and development expense in connection with the IDT License Agreement. There are no milestones probable as of December 31, 2022 and 2021; therefore, no milestone payments have been recognized in the years ended December 31, 2022 and 2021.
Master Development and Manufacturing Services Agreement (for cell therapy services)
In November 2022, we entered into a master development and manufacturing services agreement, with the WuXi Agreement, pursuant to which we may engage WuXi, at our discretion, to provide services under separate work orders for cell therapy services. To date, pursuant to the WuXi Agreement, we have entered into three work orders for cell therapy services. Following our decision to discontinue the development of nula-cel, we do not intend to enter into new work orders under the WuXi Agreement.
Bayside Lease
On December 16, 2021, the Company entered into a lease agreement with Bayside Area Development, LLC (“Bayside”) for 85,165 square feet of office and laboratory space at 233 E Grand Avenue, South San Francisco (the “Bayside Lease Agreement”). Pursuant to the Bayside Lease Agreement, the Company is expected to commence the lease on or before September 15, 2023 with a total term of 120 months. Future minimum lease payments under the Bayside Lease Agreement total $81.0 million, which does not include lease payments related to the Company’s one-time option to extend for an additional ten years.
130
As of December 31, 2022 and 2021, the Company posted a security deposit in the amount of $1.6 million in the form of a letter of credit in connection with the Bayside Lease Agreement, which is classified as restricted cash, non-current on the balance sheet. In addition, the lessor provided for a tenant improvement allowance of up to $14.9 million, which is expected to be fully utilized. As of December 31, 2022, the Company had recognized $12.2 million of tenant improvements, which was recorded as construction-in-progress and included within property and equipment, net on the Company’s balance sheets.
As of December 31, 2022 and 2021, there was no right-of-use asset or lease liability recorded on the balance sheet for the Bayside Lease Agreement as the Company has not yet obtained possession of the space and the lease has not yet commenced.
7. Commitments and Contingencies
Research and Development Agreements
The Company enters into contracts in the normal course of business with CROs for clinical trials, with CMOS or other vendors for preclinical studies, supplies and other services and products for operating purposes. These contracts generally provide for termination on notice or may have a potential termination fee if a purchase order is cancelled within a specified time. As of December 31, 2022 and 2021, there were no amounts accrued related to termination and cancellation charges as the Company has not determined cancellation to be probable.
License Agreements
The Company entered into the license agreements (Note 6), pursuant to which the Company is required to pay certain cash milestones contingent upon the achievement of specific events. No such milestones were achieved or probable as of December 31, 2022 and 2021. The Company is required to pay royalties on sales of products developed under this agreement. All products are in development as of December 31, 2022 and 2021 and no such royalties were due.
Legal Contingencies
From time to time, the Company may become involved in legal proceedings arising from the ordinary course of business. The Company records a liability for such matters when it is probable that future losses will be incurred and that such losses can be reasonably estimated. Significant judgment by the Company is required to determine both probability and the estimated amount. Management is currently not aware of any legal matters that could have a material adverse effect on the Company’s financial position, results of operations or cash flows.
Guarantees and Indemnifications
In the normal course of business, the Company enters into agreements that contain a variety of representations and provide for general indemnification. Its exposure under these agreements is unknown because it involves claims that may be made against the Company in the future. To the extent permitted under Delaware law, the Company has agreed to indemnify its directors and officers for certain events or occurrences while the director or officer is, or was serving, at a request in such capacity. To date, the Company has not paid any claims or been required to defend any action related to its indemnification obligations. As of December 31, 2022 and 2021, the Company did not have any material indemnification claims that were probable or reasonably possible and consequently has not recorded related liabilities.
8. Operating Leases
In February 2016, the Financial Accounting Standards Board (“FASB”) issued Accounting Standards Update No. 2016-02, Leases (“ASC 842”). Under ASC 842, the Company determines if an arrangement is a lease at inception. Lease liabilities and their corresponding right-of-use assets are recorded based on the present value of lease payments over the expected lease term. In determining the present value of lease payments, the Company uses its incremental borrowing rate based on the information available at the lease commencement date if the rate implicit in the lease is not readily determinable. The new standard requires lessees to apply a dual approach, classifying leases as either finance or operating leases based on the principle of whether or not the lease is effectively a financed purchase by the lessee. This classification will determine whether lease expense is recognized based on an effective interest method or on a straight-line basis over the term of the lease. A lessee is also required to record right-of-use assets and lease liabilities for all leases with a term of greater than 12 months regardless of their classification. Leases with a term of 12 months or less may be accounted for similar to existing guidance for operating leases today and are not recorded on the Company’s balance sheet. The Company early adopted the new standard as of January 1, 2021 on a modified retrospective basis with no cumulative adjustment to accumulated deficit. The Company elected to take the practical expedient to not separate lease and non-lease components as part of the adoption. Lease agreements entered into after the adoption of Topic 842 that include lease and non-lease components are accounted for as a single lease component. Beginning on January 1, 2021, any of the Company’s new operating leases, excluding those with terms less than 12 months, will be discounted and recorded as assets and liabilities on the Company’s balance sheet.
131
As of December 31, 2022, the current and non-current portions of the total liability for operating leases was $4.0 million $1.7 million, respectively. As of December 31, 2022, the weighted average remaining lease term on the operating lease is 19 months. The weighted average incremental borrowing rate used to determine the operating lease liabilities included on the balance sheet was 8.5%.
Facility leases
In April 2020, the Company entered into a one-year lease agreement for its headquarter facility located in South San Francisco, California with a significant portion of the premises allocated to the research lab. Due to the COVID-19 pandemic, the use of the entire facility was temporarily designated to research, and as such, all associated costs were expensed as research and development. In addition to payment of base rent, the Company is also required to pay property taxes, insurance, and common area expenses. The Company records lease expense on a straight-line basis over the term of the lease. The original term of the lease was from May 11, 2020 to June 30, 2021 with an option to renew. In March 2021, the Company entered into an amendment to the lease agreement and extended the term of the lease to September 30, 2021. The lease expired without further renewal and was terminated on September 30, 2021.
On January 27, 2021, the Company entered into a new lease agreement for office and lab space in South San Francisco, California that included two office suites. The lease terms for the two office suites commenced during and , respectively. The term of the lease is 44 months for the first office suite and 43 months for the second office suite with an option to extend the term for an additional two years on the same terms and conditions. This option to extend the lease term was not determined to be reasonably certain and therefore has not been included in the Company’s calculation of the associated operating lease liability under ASC 842. The corresponding right-of-use assets and lease liabilities related to the two office suites were recorded on the Company’s balance sheet upon the lease commencement date, which was the date the Company was deemed to have obtained control of the premises.
On November 10, 2021, the Company entered a sublease agreement for office and lab space located at Brisbane, California. The sublease expires on December 6, 2023. The corresponding right-of-use assets and lease liabilities related to the sublease were recorded on the Company’s balance sheet upon the lease commencement date, which was the date the Company was deemed to have obtained control of the premises.
As of December 31, 2022, the Company had operating lease right-of-use assets of $4.2 million and operating lease liabilities of $4.5 million related to the office suite leases recorded on its balance sheet.
Embedded leases
The Company evaluated its vendor contracts to identify embedded leases, if any, and determined that two agreements with contract manufacturing suppliers constituted a lease under ASC 842 as the Company has the right to substantially all of the economic benefits from the use of the asset and can direct the use of the asset.
On May 10, 2021 and August 30, 2021, the Company and LCGM entered into the LCGM MSA and SOW #3, respectively, for the exclusive use of a manufacturing suite at the LCGM facility. Pursuant to the terms of SOW #3, LCGM agreed to provide the Company with certain dedicated space for the clinical manufacturing, release testing, and product release in the Company’s Phase 1 clinical trial to treat Sickle Cell Disease. The Company concluded that the agreement contains an embedded lease as the Company controls the use of a dedicated manufacturing suite and the equipment therein. The agreement includes fixed lease payments of $5.6 million through April 30, 2023, the expiration date of SOW #3.
The Company and Explora BioLabs, Inc. (“Explora”) entered into a License Service Agreement and Master Services Agreement (together, the “Explora Agreements”) on November 17, 2021 and December 16, 2021, respectively. Pursuant to the terms of the Explora Agreements, Explora agreed to provide a certain dedicated space to perform in vitro or in vivo studies, obtain or house research animals, and provide scientific or technical consultation to the Company. The Company concluded that the agreement contains an embedded lease as the Company controls the use of a dedicated manufacturing suite and the equipment therein. The Explora Agreements contain fixed lease payments of $0.7 million through .
As of December 31, 2022, the Company had operating lease right-of-use assets of $1.4 million and operating lease liabilities of $1.2 million related to the embedded leases recorded on its balance sheet related to the embedded leases.
132
Operating Lease Obligations
As of December 31, 2022, the future minimum lease payments for the Company’s operating leases for each of the years ending December 31 were as follows (in thousands):
|
|
Amount |
|
||
2023 |
|
$ |
|
4,331 |
|
2024 |
|
|
|
1,468 |
|
2025 |
|
|
|
375 |
|
Thereafter |
|
|
|
— |
|
Total undiscounted lease payments |
|
|
|
6,174 |
|
Present value adjustment |
|
|
|
(380 |
) |
Total net lease liabilities |
|
$ |
|
5,794 |
|
Lease expense was $6.7 and $1.9 million for the years ended December 31, 2022 and 2021, respectively.
Under the terms of the remaining lease agreements, the Company is also responsible for certain variable lease payments that are not included in the measurement of the lease liability. Variable lease payments for operating leases were $1.3 and $0.2 million for the years ended December 31, 2022 and 2021, respectively, including non-lease components such as common area maintenance fees, taxes, and insurance.
The following information represents supplemental disclosure for the statement of cash flows related to the operating leases (in thousands):
|
|
December 31, 2022 |
|
||
Cash paid for amounts included in the measurement of lease liabilities |
|
|
|
|
|
Operating cash flows under operating leases |
|
$ |
|
6,172 |
|
9. Redeemable Convertible Preferred Stock
In June 2021, immediately prior to the completion of the Company’s IPO (Note 1), all outstanding shares of redeemable convertible preferred stock were automatically converted into 30,761,676 shares of common stock. Upon conversion into common stock, the carrying value of the redeemable convertible preferred stock of $260.5 million was reclassified to equity.
Series A Redeemable Convertible Preferred Stock
In June 2020, the Company issued 10,000,000 shares of its Series A redeemable convertible preferred stock at a price of $1.00 per share for gross cash proceeds of $10.0 million and issued 5,019,949 shares of its Series A redeemable convertible preferred stock upon the conversion of the outstanding convertible note and accrued interest.
In connection with the initial issuance of the shares of its Series A redeemable convertible preferred stock, the Company had an obligation to sell and the holders had the obligation to purchase the additional 30,000,000 shares of Series A redeemable convertible preferred stock at $1.00 per share upon the achievement of certain milestones as determined by the Board and approved by at least one of the investors, or upon the waiver of such milestones by the holders of at least 75% of the outstanding shares of Series A redeemable convertible preferred stock, in two equal tranches of $15.0 million each. The Company determined that the obligation to sell additional shares is a freestanding financing instrument and a liability. The Company estimated the fair value of the liability to be $3.3 million and recorded it as a reduction to redeemable convertible preferred stock and as a derivative redeemable convertible preferred stock tranche liability in its balance sheet at the issuance date.
In December 2020, the requisite holders waived the second tranche milestone event and the Company issued 15,000,000 shares of its Series A redeemable convertible preferred stock for gross cash proceeds of $15.0 million. The redeemable convertible preferred stock tranche liability related to the second tranche shares was remeasured to fair value of $29.1 million and reclassified to redeemable convertible preferred shares upon the settlement.
In connection with the issuance of Series A redeemable convertible preferred stock, in the year ended December 31, 2020, the Company incurred issuance costs of $0.2 million. As of December 31, 2020, the redeemable convertible preferred stock tranche liability related to the third tranche shares was remeasured at fair value of $29.1 million and continued to be reported in current liabilities.
The Company settled the third tranche in February 2021 and issued 15,000,000 shares of its Series A redeemable convertible preferred stock for gross cash proceeds of $15.0 million. The Company recognized a total of $54.8 million as other loss in the statements of operations and comprehensive loss related to the changes in the fair value of the redeemable convertible preferred stock tranche liabilities during the year ended December 31, 2020.
Prior to the closing of the third tranche of the Series A preferred stock financing in February 2021, the remaining tranche liability was remeasured at a fair value of $39.4 million. The Company recognized a loss of $10.3 million in the statements of operations and
133
comprehensive loss related to the change in the fair value of the redeemable convertible preferred stock tranche liability during the year ended December 31, 2021.
In connection with the closing of the third tranche of Series A redeemable convertible preferred stock, the Company incurred issuance costs of $4.0 thousand during the year ended December 31, 2021.
Series B Redeemable Convertible Preferred Stock
In March 2021, the Company issued 29,792,487 shares of the Series B redeemable convertible preferred stock at $5.06 per share for gross cash proceeds of $150.7 million. The Company incurred issuance costs of $0.2 million.
10. Common Stock
As of December 31, 2022 and 2021, the Company was authorized to issue 300,000,000 shares of its common stock with $0.00001 par value per share. Each share of the Company’s common stock is entitled to one vote. In connection with the IPO in June 2021, all outstanding shares of redeemable convertible preferred stock were converted into 30,761,676 shares of common stock. The IPO closed on June 29, 2021, pursuant to which the Company issued and sold 14,000,000 shares of its common stock at a public offering price of $17.00 per share.
134
On June 29, 2021, the underwriters also exercised their option to purchase an additional 2,100,000 shares of common stock at the IPO price, less the underwriting discounts and commissions. The closing of the offering of the additional shares occurred on July 2, 2021. The Company issued and sold 2,100,000 shares of its common stock at a public offering price of $17.00 per share.
Shares Reserved for Future Issuance
As of December 31, 2022 and 2021, the Company reserved common stock for future issuances as follows:
|
|
December 31, 2022 |
|
|
December 31, 2021 |
|
||
Outstanding stock option awards |
|
|
7,755,303 |
|
|
|
5,056,743 |
|
Shares available for future stock option grants |
|
|
5,382,907 |
|
|
|
4,454,004 |
|
ESPP shares available for future grants |
|
|
754,951 |
|
|
|
524,106 |
|
Total shares reserved for future issuance |
|
|
13,893,161 |
|
|
|
10,034,853 |
|
Founders’ and Investor’s Restricted Common Stock
In March 2020, the Board approved and in April 2020, the Company issued 6,081,413 shares of its common stock to its founders and 2,467,104 shares of its common stock to its investor at the purchase price of $0.00002 per share. As of December 31, 2020, the investor’s shares were fully vested and a portion of the shares issued were subject to the Company’s option to repurchase per the Stanford Adjustment Repurchase Right, as described below.
The shares of the Company’s common stock issued to its founders for their services as an employee, advisor, or consultant vest monthly over four years with one year cliff from the vesting commencement date. The vesting commencement date was the date of the initial closing of the Series A preferred stock financing or June 24, 2020. Pursuant to the restricted stock purchase agreements with each of the founders, the vesting of the founders’ common stock shares could be accelerated upon the occurrence of certain events, including signing of the term sheet for the license with Stanford, a change in control, or if the founder’s service is terminated by the Company without cause. The Company signed the term sheet with Stanford in June 2020, and as a result, an aggregate of 912,212 shares of founders’ common stock vested pursuant to the acceleration terms.
If a founder terminates the service relationship with the Company during the vesting period, the Company may repurchase any unvested restricted common stock at the price per share equal to the lower of (i) the original purchase price, subject to adjustment in the event of any reorganization, recapitalization, reclassification, stock dividend, stock split, reverse stock split, or (ii) the current fair market value as of the date the Company elects to exercise its Stanford Adjustment Repurchase Right, as described below. The repurchase right lapses in 180 days after the termination of the founder’s service or employment. During the vesting term, holders of founders’ common stock awards are deemed to be common stockholders and have the right to receive dividends and voting rights.
The founders’ shares of common stock are also subject to the Company’s option to repurchase per the Stanford Adjustment Repurchase Right, as described below.
The Company accounts for shares issued to founders as equity compensation awards and the estimated fair value at the grant date was minimal. 1,938,430 and 3,230,746 shares of founders’ common stock awards were unvested and expected to vest in 1.5 years and 2.5 years as of December 31, 2022 and 2021, respectively.
Stanford Adjustment Repurchase Right
Upon the issuance of shares of common stock to Stanford pursuant to the License Agreement, as discussed in Note 5, the Company has a right to repurchase from each founder and an investor a number of shares of common stock equal to the number of shares issued to Stanford multiplied the applicable number of shares issued to the founder or investor, as applicable, divided by 7,273,848 shares (a fully diluted number of shares of the Company at the end of March 2020, after founders and the investor’s shares were approved by the board of directors). The Stanford Adjustment Repurchase Right may be exercised by the Company within six months following the date of the issuance of the shares of common stock to Stanford. The repurchase price per share is equal to the lower of (i) the purchase price, subject to adjustment in the event of any reorganization, recapitalization, reclassification, etc., or (ii) the current fair market value as of the date the Company elects to exercise its Stanford Adjustment Repurchase Right.
On May 7, 2021, the Company issued an aggregate of 640,861 shares of the Company’s common stock to Stanford and certain individuals designated by Stanford in consideration for rights granted to the Company under the Company’s exclusive license agreement.
On June 18, 2021, the Company exercised its right to repurchase an aggregate of 624,845 shares from each founder and investor under the Stanford Adjustment Repurchase Right. As of December 31, 2022, the Company has not exercised the right to purchase the remaining 16,016 shares.
The Company accounts for the founders and investor’s shares of restricted common stock as equity share-based awards.
135
11. Equity Incentive Plans
2020 Stock Option and Grant Plan
The Company grants share-based awards under the 2020 Stock Option Plan, as amended (the “2020 Plan”). The Company may grant under the 2020 Plan incentive stock options, nonqualified stock options, restricted stock awards, restricted stock units and other share-based awards to the Company’s officers, employees, directors and consultants. Options under the 2020 Plan may be granted for periods of up to 10 years and at prices no less than 100.0% of the estimated fair value of the shares on the date of grant as determined by the Board, provided, however, that the exercise price of an incentive stock option granted to a 10.0% stockholder shall not be less than 110.0% of the estimated fair value of the shares on the date of grant and the option is not exercisable after the expiration of five years from the date of grant. Options generally vest monthly over four years with or without one year cliff vesting. Per the 2020 Plan, granted options maybe early exercised prior to vesting and the Company will issue shares of restricted stock upon the early exercise with vesting terms consistent with the original grant. Upon completion of the Company’s IPO, the remaining shares available for issuance under the 2020 Plan were retired.
2021 Stock Option and Grant Plan
In June 2021, the Company’s Board of Directors approved the 2021 Stock Option and Incentive Plan (the “2021 Plan”) that became effective immediately prior to the date when the Company’s prospectus was declared effective by the SEC on June 24, 2021. The Company initially reserved 5,636,000 shares of common stock for issuance of awards under the 2021 Plan. The 2021 Plan provides that the number of shares reserved and available for issuance under the plan will automatically increase each January 1, beginning on January 1, 2022, by 5% of the outstanding number of shares of the Company’s common stock on the immediately preceding December 31, or such lesser number of shares as determined by the Company’s compensation committee. On January 1, 2022, the number of shares of common stock available under the 2021 Plan increased by 2,900,541 shares pursuant to this evergreen provision of the 2021 Plan. The option exercise price of each option will be determined by the Company’s compensation committee but generally may not be less than 100% of the fair market value of the Company’s common stock on the date of grant. The term of each option will be fixed by the Company’s compensation committee and may not exceed ten years from the date of grant. The grant date fair value of all awards made under the 2021 Plan and all other cash compensation paid by the Company to any non-employee director for services as a non-employee director in any calendar year may not exceed $1.0 million for the first year of service and $750.0 thousand for each year of service thereafter.
As of December 31, 2022, there were 5,382,907 shares available for future issuance under the 2021 Plan.
Restricted Stock Awards
During the year ended December 31, 2020, the Company issued 832,983 shares as restricted stock awards under the 2020 Plan. The purchase price of the restricted common stock awards was fair value as determined by the Board at the issuance date. The shares vest monthly over four years with the one-year cliff vesting from the grant date. Upon termination of employment, the Company has the right to repurchase any unvested restricted shares. The repurchase price for unvested shares of common stock will be the lower of (i) the fair market value on the date of repurchase or (ii) their original purchase price. There were no shares issued during the years ended December 31, 2022 and 2021.
The Company accounted for restricted stock awards as early exercised options and recognized a liability in other liabilities when cash was received for the purchase of shares of restricted stock awards. As shares of restricted stock awards vest, the Company reclassified the liability to common stock and additional paid in capital. As of December 31, 2022 and 2021, the Company recorded a minimal liability for restricted stock awards included in accrued expenses and other current liabilities.
There were 5,140 shares of restricted stock award shares canceled and repurchased as of December 31, 2022. No restricted stock award shares were cancelled or repurchased as of December 31, 2021. There were 553,443 and 345,966 shares of restricted stock vested as of December 31, 2022 and 2021, respectively.
Employee Stock Purchase Plan
In June 2021, the Company’s board of directors and stockholders approved the 2021 Employee Stock Purchase Plan (the “2021 ESPP”) which became effective upon the IPO. The 2021 ESPP is intended to qualify as an employee stock purchase plan under Section 423 of the Internal Revenue Code of 1986, as amended, and is administered by the Company’s board of directors and the Compensation Committee of the board of directors. Under the 2021 ESPP, 564,000 shares of the Company’s common stock have been initially reserved for employee purchases of the Company’s common stock. The 2021 ESPP allows eligible employees to purchase shares of the Company’s common stock at a discount through payroll deductions of up to 15% of their eligible compensation. At the end of each offering period, employees are able to purchase shares at 85% of the lower of the fair market value of the Company’s common stock at the beginning of the offering period or at the end of each applicable purchase period. The first purchase period commenced upon the
136
completion of the Company’s IPO and ended on November 30, 2021 and the subsequent offering periods commenced on December 1, 2021, June 1, 2022, and December 1, 2022. The Company recorded $0.1 million in accrued liabilities as of December 31, 2022.
Effective January 1, 2022, the number of shares of common stock available under the 2021 ESPP increased by 564,000 shares pursuant to the evergreen provision of the 2021 ESPP.
|
|
Year Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Expected volatility |
|
73.00% - 75.00% |
|
|
72.00% - 74.00% |
|
||
Expected dividend yield |
|
|
0 |
% |
|
|
0 |
% |
Expected term (in years) |
|
0.5 |
|
|
0.44 - 0.5 |
|
||
Risk-free interest rate |
|
0.10% - 4.65% |
|
|
0.10% - 1.00% |
|
||
Incentive Stock Options and Nonqualified Stock Options
Stock options issued under the 2020 Plan and 2021 Plan, generally, vest over a four-year period and expire ten years from the date of grant. Certain options provide for accelerated vesting if there is a change in control, as defined in the individual award agreements.
The Company used the Black-Scholes option pricing model to estimate stock-based compensation expense for stock option awards granted during the periods presented, with the following assumptions.
|
|
Year Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Expected volatility |
|
74.00% - 75.00% |
|
|
72.00% - 77.74% |
|
||
Expected dividend yield |
|
|
0 |
% |
|
|
0 |
% |
Expected term (in years) |
|
5.97 - 6.01 |
|
|
5.48 - 6.04 |
|
||
Risk-free interest rate |
|
1.91% - 4.23% |
|
|
0.56% - 1.34% |
|
||
137
A summary of option activity under the 2020 Plan and the 2021 Plan during the year ended December 31, 2022 is as follows:
|
|
Number of |
|
|
Weighted- |
|
|
Weighted- |
|
|
Aggregate |
|
||||
Outstanding as of December 31, 2021 |
|
|
5,056,743 |
|
|
$ |
8.77 |
|
|
|
9.2 |
|
|
$ |
18,503 |
|
Options granted - 2021 Plan |
|
|
3,292,702 |
|
|
$ |
7.91 |
|
|
|
|
|
|
|
||
Options exercised |
|
|
(67,196 |
) |
|
$ |
0.30 |
|
|
|
|
|
|
|
||
Options cancelled |
|
|
(526,946 |
) |
|
$ |
8.99 |
|
|
|
|
|
|
|
||
Outstanding as of December 31, 2022 |
|
|
7,755,303 |
|
|
$ |
8.47 |
|
|
|
8.7 |
|
|
$ |
794 |
|
Exercisable |
|
|
2,330,389 |
|
|
$ |
8.74 |
|
|
|
8.3 |
|
|
$ |
388 |
|
Vested and expected to vest as of December 31, 2022 |
|
|
7,755,303 |
|
|
$ |
8.47 |
|
|
|
8.7 |
|
|
$ |
794 |
|
Aggregate intrinsic value represents the difference between the fair value of the underlying common stock and the exercise price as of December 31, 2022. The weighted-average grant date fair value of options granted during the year ended December 31, 2022 was $5.21 per share. The intrinsic value of the stock options exercised was $0.2 and $5.0 million for the years ended December 31, 2022 and 2021, respectively.
Early Exercise of Stock Options
The terms of the 2020 Plan permit the exercise of options granted prior to vesting, subject to required approvals. The unvested shares are subject to the repurchase right upon termination of employment at the original purchase price. The repurchase right lapses in 180 days after the termination of the employee’s employment. Shares purchased by employees pursuant to the early exercise of stock options are not deemed, for accounting purposes, to be issued until those shares vest according to their respective vesting schedules. Cash received for early exercised stock options is recorded as other liabilities on the balance sheet and is reclassified to common stock and additional paid-in capital as such shares vest. During the years ended December 31, 2022 and 2021, the Company repurchased 189,414 and 138,596 shares that were previously early exercised.
At December 31, 2022 and 2021, 554,695 and 1,195,631 shares, respectively, remained subject to the right of repurchase as a result of the early exercised stock options. The remaining liability related to early exercised shares as of December 31, 2022 and 2021 was $0.1 and $0.3 million, respectively, and was recorded in accrued expenses and other current liabilities, respectively, in the balance sheets.
Stock-Based Compensation Expense
The following table presents the components of stock-based compensation expense for the Company’s stock-based awards for the periods presented (in thousands):
|
|
Year Ended |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Restricted stock awards and founders’ common stock awards |
|
$ |
11 |
|
|
$ |
9 |
|
ESPP |
|
|
391 |
|
|
|
215 |
|
Stock options |
|
|
13,132 |
|
|
|
7,647 |
|
Total stock-based compensation expense |
|
$ |
13,534 |
|
|
$ |
7,871 |
|
The above stock-based compensation expense also includes the expenses of $2.2 and $0.3 million related to stock options issued to non-employees during the years ended December 31, 2022 and 2021, respectively.
The following table presents the classification of stock-based compensation expense for the Company’s stock-based awards for the periods presented (in thousands):
|
|
Year Ended |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Research and development expenses |
|
$ |
5,317 |
|
|
$ |
2,685 |
|
General and administrative expenses |
|
|
8,217 |
|
|
|
5,186 |
|
Total stock-based compensation expense |
|
$ |
13,534 |
|
|
$ |
7,871 |
|
138
As of the years ended December 31, 2022 and 2021, there was $31.0 and $30.5 million, respectively, of unrecognized stock-based compensation expense related to the employee and non-employee awards, which is expected to be recognized over a weighted-average period of 2.6 years and 3.3 years, respectively.
12. Net Loss Per Share Attributable to Common Stockholders
The following table sets forth the computation of basic and diluted net loss per share attributable to common stockholders, which excludes shares which are legally outstanding, but subject to repurchase by the Company (in thousands, except share and per share amounts):
|
|
Year Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Numerator: |
|
|
|
|
|
|
||
Net loss |
|
$ |
(101,052 |
) |
|
$ |
(70,760 |
) |
Denominator: |
|
|
|
|
|
|
||
Weighted-average common shares outstanding |
|
|
58,111,437 |
|
|
|
35,121,114 |
|
Less: weighted-average unvested restricted shares and shares subject to repurchase |
|
|
(3,237,762 |
) |
|
|
(6,201,859 |
) |
Weighted-average shares used to compute basic and diluted net loss per share attributable to common stockholders |
|
|
54,873,675 |
|
|
|
28,919,255 |
|
Net loss per share attributable to common stockholders — basic and |
|
$ |
(1.84 |
) |
|
$ |
(2.45 |
) |
Anti-dilutive Outstanding Shares or Equivalents
The following outstanding options, unvested shares, and ESPP shares were excluded (as common stock equivalents) from the computation of diluted net loss per common share for the periods presented as their effect would have been antidilutive (in thousands):
|
|
Year Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Options to purchase common stock |
|
|
7,755,303 |
|
|
|
5,056,743 |
|
Common stock subject to vesting or repurchase |
|
|
2,767,526 |
|
|
|
4,774,798 |
|
Employee Stock Purchase Plan shares |
|
|
168,080 |
|
|
|
99,252 |
|
Total |
|
|
10,690,909 |
|
|
|
9,930,793 |
|
139
13. Income Taxes
No provision for income taxes was recorded for the years ended December 31, 2022 and 2021. The Company has incurred net operating losses only in the United States since its inception. The Company has not reflected any benefit of such net operating loss carryforwards in the financial statements.
A reconciliation of the U.S. federal statutory income tax rate to the Company’s effective income tax rate was as follows:
|
|
Year Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Federal statutory income tax rate ate |
|
|
21.00 |
% |
|
|
21.00 |
% |
State taxes |
|
|
1.10 |
|
|
1.94 |
|
|
Others |
|
|
(0.69 |
) |
|
|
(0.26 |
) |
Research and development credits |
|
|
1.11 |
|
|
|
1.01 |
|
Cancellation of debt income |
|
|
— |
|
|
|
— |
|
Tranche liability |
|
|
— |
|
|
|
(3.06 |
) |
Interest expense |
|
|
— |
|
|
|
— |
|
Stock-based compensation |
|
|
(0.97 |
) |
|
|
(0.96 |
) |
Change in valuation allowance |
|
|
(21.55 |
) |
|
|
(19.67 |
) |
Provision for taxes |
|
|
0.00 |
% |
|
|
0.00 |
% |
Net deferred tax assets and liabilities consisted of the following (in thousands):
|
|
Year Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Deferred tax assets: |
|
|
|
|
|
|
||
Net operating losses - non-current |
|
$ |
15,940 |
|
|
$ |
12,006 |
|
Capitalized R&D |
|
|
13,815 |
|
|
|
— |
|
General business credit - non-current |
|
|
5,699 |
|
|
|
2,682 |
|
Operating lease right-of-use assets |
|
|
1,220 |
|
|
|
2,368 |
|
Stock based compensation |
|
|
1,703 |
|
|
|
784 |
|
Accruals and reserves |
|
|
735 |
|
|
|
525 |
|
Fixed assets |
|
|
170 |
|
|
|
396 |
|
Other |
|
|
3 |
|
|
|
2 |
|
Gross deferred tax assets |
|
|
39,285 |
|
|
|
18,763 |
|
Valuation allowance |
|
|
(38,110 |
) |
|
|
(16,332 |
) |
Net deferred tax assets |
|
|
1,175 |
|
|
|
2,431 |
|
Fixed asset basis |
|
|
— |
|
|
|
— |
|
Operating lease liabilities |
|
|
(1,175 |
) |
|
|
(2,431 |
) |
Other |
|
|
— |
|
|
|
— |
|
Gross deferred tax liabilities |
|
|
(1,175 |
) |
|
|
(2,431 |
) |
Valuation allowance |
|
$ |
— |
|
|
$ |
— |
|
Net operating losses and tax credit carryforwards were as follows as of December 31, 2022 (dollars in thousands):
|
|
Year Ended December 31, 2022 |
||||
|
|
Amount |
|
|
Expiration Years |
|
Net operating losses, federal (starting from January 1, 2018) |
|
$ |
75,730 |
|
|
Do Not Expire |
Net operating losses, state |
|
|
3,195 |
|
|
2039 - 2042 |
Tax credits, federal |
|
|
5,309 |
|
|
2041 - 2042 |
Tax credits, state |
|
|
592 |
|
|
Do Not Expire |
140
Utilization of the net operating loss carryforwards and research credit carryforwards may be subject to an annual limitation due to the ownership percentage change limitations provided by the Internal Revenue Code, as amended, (“IRC”), and similar state provisions. Annual limitations may result in the expiration of the net operating losses and tax credit carryforwards before they are utilized. The Company did not perform an IRC Section 382 analysis and any previous ownership changes may result in a limitation that will reduce the total amount of net operating loss and tax credit carryforwards disclosed that can be utilized. Subsequent ownership changes may affect the limitation in future years.
During the years ended December 31, 2022 and 2021, the Company recorded a full valuation allowance on federal and state deferred balances since management does not forecast the Company to be in a profitable position in the near future. Changes in the valuation allowance for deferred tax assets during the years ended December 31, 2022 and 2021 related primarily to the increases in net operating loss carryforwards and research and development tax credit carryforwards and were as follows (in thousands):
|
|
Year Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Valuation allowance at the beginning of the year |
|
$ |
16,332 |
|
|
$ |
2,414 |
|
Increases recorded to income tax provision |
|
|
21,778 |
|
|
|
13,918 |
|
Valuation allowance at the end of the year |
|
$ |
38,110 |
|
|
$ |
16,332 |
|
The Company’s U.S. federal and state income tax returns are generally subject to tax examinations for the tax years from inception through December 31, 2021. There are currently no pending income tax examinations. To the extent the Company has tax attribute carryforwards, the tax years in which the attribute was generated may still be adjusted upon examination by the Internal Revenue Service and state tax authorities to the extent utilized in a future period.
A reconciliation of the beginning and ending amount of unrecognized tax benefits is as follows (in thousands):
|
|
Year Ended December 31, |
|
|||||
|
|
2022 |
|
|
2021 |
|
||
Balance at beginning of year |
|
$ |
1,407 |
|
|
$ |
— |
|
Additions based on tax positions related to current year |
|
|
1,862 |
|
|
|
1,258 |
|
Increase (reduction) for prior period positions |
|
|
(568 |
) |
|
|
149 |
|
Unrecognized tax benefit-December 31 |
|
$ |
2,701 |
|
|
$ |
1,407 |
|
The entire amount of the unrecognized tax benefits would not impact the Company’s effective tax rate if recognized. The Company’s policy is to record interest and penalties related to income taxes as part of its income tax provision. The Company has elected to include interest and penalties as a component of tax expense. During the years ended December 31, 2022 and 2021, the Company did not recognize accrued interest and penalties related to unrecognized tax benefits. The Company does not anticipate that the amount of existing unrecognized tax benefits will significantly increase or decrease during the next 12 months.
14. Subsequent Events
In February 2023, we announced a workforce reduction plan (the “Plan”) intended to realign our investments to accelerate our growth strategy and further optimize our operations and cost structure. The Plan is expected to result in reductions to our headcount of approximately 50% during 2023. In connection with the Plan, we currently estimate that we will incur charges consisting primarily of cash termination benefits and other employee-related costs. We are continuing to evaluate the amount of these charges and expect that the majority of these charges will be recognized during the second quarter of 2023.
141
Item 9. Changes in and Disagreements with Accountants on Accounting and Financial Disclosure.
None.
Item 9A. Controls and Procedures.
Evaluation of Disclosure Controls and Procedures
Our management, with the participation of our Chief Executive Officer and our Chief Financial Officer, has evaluated the effectiveness of our disclosure controls and procedures (as defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act of 1934, as amended (the “Exchange Act”) as of the end of the period covered by this Annual Report. Based upon that evaluation, our Chief Executive Officer and Chief Financial Officer have concluded that, as of December 31, 2022, our disclosure controls and procedures were effective to provide reasonable assurance that information required to be disclosed by us in the reports that we file or submit under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms and to provide reasonable assurance that such information is accumulated and communicated to our management, including our Chief Executive Officer and Chief Financial Officer, as appropriate, to allow timely decisions regarding required disclosure.
Management’s Annual Report on Internal Control over Financial Reporting
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. Internal control over financial reporting is defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act as a process designed by, or under the supervision of, a company’s principal executive officer and principal financial officer, or persons performing similar functions, and effected by a company’s board of directors, management, and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Under the supervision of and with the participation of our principal executive officer and principal financial officer, our management assessed the effectiveness of our internal control over financial reporting as of December 31, 2022 based on the criteria set forth by the Committee of Sponsoring Organizations of the Treadway Commission in Internal Control—Integrated Framework (2013 framework). Based on this assessment, management concluded that our internal control over financial reporting was effective as of December 31, 2022.
This Annual Report does not include an attestation report of the Company's independent registered public accounting firm due to a transition period established by rules of the SEC for newly public companies.
Changes in Internal Control Over Financial Reporting
There were no changes in our internal control over financial reporting (as defined in Rules 13a-15(f) and 15d-15(f) under the Exchange Act) that occurred during the year ended December 31, 2022 that has materially affected, or is reasonably likely to materially affect, our internal control over financial reporting.
Inherent Limitations on Effectiveness of Controls
Our management, including our Chief Executive Officer and Chief Financial Officer, do not expect that our disclosure controls or our internal control over financial reporting will prevent all errors and all fraud. A control system, no matter how well conceived and operated, can provide only reasonable, not absolute, assurance that the objectives of the control system are met. Further, the design of a control system must reflect the fact that there are resource constraints, and the benefits of controls must be considered relative to their costs. Because of the inherent limitations in all control systems, no evaluation of controls can provide absolute assurance that all control issues and instances of fraud, if any, have been detected. These inherent limitations include the realities that judgments in decision-making can be faulty, and that breakdowns can occur because of a simple error or mistake. Additionally, controls can be circumvented by the individual acts of some persons, by collusion of two or more people or by management override of the controls. The design of any system of controls is also based in part upon certain assumptions about the likelihood of future events, and there can be no assurance
142
that any design will succeed in achieving its stated goals under all potential future conditions, over time, controls may become inadequate because of changes in conditions, or the degree of compliance with policies or procedures may deteriorate. Because of the inherent limitations in a cost-effective control system, misstatements due to error or fraud may occur and not be detected.
Item 9B. Other Information.
None.
Item 9C. Disclosure Regarding Foreign Jurisdictions that Prevent Inspections.
Not applicable.
143
PART III
Item 10. Directors, Executive Officers and Corporate Governance.
The information required by this Item 10 will be included in our definitive proxy statement to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2022 with respect to our 2023 Annual Meeting of Stockholders, which information is incorporated herein by reference.
Item 11. Executive Compensation.
The information required by this Item 11 will be included in our definitive proxy statement to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2022 with respect to our 2023 Annual Meeting of Stockholders, which information is incorporated herein by reference.
Item 12. Security Ownership of Certain Beneficial Owners and Management and Related Stockholder Matters.
The information required by this Item 12 will be included in our definitive proxy statement to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2022 with respect to our 2023 Annual Meeting of Stockholders, which information is incorporated herein by reference.
The information required by this Item 13 will be included in our definitive proxy statement to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2022 with respect to our 2023 Annual Meeting of Stockholders, which information is incorporated herein by reference.
Item 14. Principal Accountant Fees and Services.
Our independent public accounting firm is Deloitte & Touche LLP, San Francisco, CA, PCAOB Auditor ID 34.
The information required by this Item 14 will be included in our definitive proxy statement to be filed with the SEC within 120 days after the end of the fiscal year ended December 31, 2022 with respect to our 2023 Annual Meeting of Stockholders, which information is incorporated herein by reference.
144
PART IV
Item 15. Exhibits, Financial Statement Schedules.
Exhibit Number |
|
Description |
3.1 |
|
|
|
|
|
3.2 |
|
|
|
|
|
4.1 |
|
|
|
|
|
4.2 |
|
|
|
|
|
4.3 |
|
|
|
|
|
10.1# |
|
|
|
|
|
10.2# |
|
|
|
|
|
10.3# |
|
|
|
|
|
10.4# |
|
|
|
|
|
10.5# |
|
|
|
|
|
10.6# |
|
|
|
|
|
10.7# |
|
|
|
|
|
10.8# |
|
|
|
|
|
10.9 |
|
|
|
|
|
10.10 |
|
|
|
|
|
10.11 |
|
|
|
|
|
10.12† |
|
|
|
|
|
10.13† |
|
|
|
|
|
145
10.14† |
|
|
|
|
|
146
10.15† |
|
|
|
|
|
10.16† |
|
|
|
|
|
10.17# |
|
|
|
|
|
10.18# |
|
|
|
|
|
10.19# |
|
|
|
|
|
10.20† |
|
|
|
|
|
10.21 |
|
|
|
|
|
10.22 |
|
|
|
|
|
10.23# |
|
|
|
|
|
10.24† |
|
|
|
|
|
21.1 |
|
|
|
|
|
23.1 |
|
Consent of Deloitte & Touche LLP, Independent Registered Public Accounting Firm. |
|
|
|
24.1 |
|
Power of Attorney (included on signature page to this Annual Report on Form 10-K) |
|
|
|
31.1 |
|
|
|
|
|
31.2 |
|
|
|
|
|
32.1* |
|
|
|
|
|
32.2* |
|
|
|
|
|
101.INS |
|
Inline XBRL Instance Document – the instance document does not appear in the Interactive Data File because XBRL tags are embedded within the Inline XBRL document |
|
|
|
101.SCH |
|
Inline XBRL Taxonomy Extension Schema Document |
|
|
|
101.CAL |
|
Inline XBRL Taxonomy Extension Calculation Linkbase Document |
|
|
|
101.DEF |
|
Inline XBRL Taxonomy Extension Definition Linkbase Document |
|
|
|
101.LAB |
|
Inline XBRL Taxonomy Extension Label Linkbase Document |
|
|
|
101.PRE |
|
Inline XBRL Taxonomy Extension Presentation Linkbase Document |
|
|
|
104 |
|
Cover Page Interactive Data File (embedded within the Inline XBRL document) |
147
_____________
† Portions of this exhibit (indicated by “[***]”) have been omitted pursuant to Item 601(b)(10) of Regulation S-K.
# Indicates a management contract or any compensatory plan, contract or arrangement.
* This certification will not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liability of that section. Such certification will not be deemed to be incorporated by reference into any filing under the Securities Act of 1933, as amended, or the Exchange Act, except to the extent specifically incorporated by reference into such filing.
The financial statements of the Registrant are included in Item 8 of this Annual Report on Form 10-K.
Schedules have been omitted because the information required to be set forth therein is not applicable or is shown in the financial statements or notes thereto.
Item 16. Form 10-K Summary.
Not applicable.
148
Signatures
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended, the Registrant has duly caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
GRAPHITE BIO, INC. |
|
|
|
|
Date: March 20, 2023 |
By: |
/s/ Josh Lehrer |
|
|
Josh Lehrer, M.D. |
|
|
President, Chief Executive Officer and Director |
Each person whose individual signature appears below hereby constitutes and appoints Josh Lehrer, M.D. and Alethia Young and each of them, with full power of substitution and resubstitution and full power to act without the other, as his or her true and lawful attorney-in-fact and agent to act in his or her name, place and stead and to execute in the name and on behalf of each person, individually and in each capacity stated below, and to file any and all amendments to this Annual Report on Form 10-K, and to file the same, with all exhibits thereto, and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing, ratifying and confirming all that said attorneys-in-fact and agents or any of them or their or his substitute or substitutes may lawfully do or cause to be done by virtue thereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, as amended, this Report has been signed below by the following persons on behalf of the Registrant in the capacities and on the dates indicated.
Signature |
|
Title |
|
Date |
|
|
|
|
|
|
|
/s/ Josh Lehrer, M.D.
Josh Lehrer, M.D. |
|
President, Chief Executive Officer and Director (Principal Executive Officer) |
|
March 20, 2023 |
|
|
|
|
|
|
|
/s/ Alethia Young
Alethia Young |
|
Chief Financial Officer (Principal Financial and Accounting Officer) |
|
March 20, 2023 |
|
|
|
|
|
|
|
/s/ Perry Karsen
Perry Karsen |
|
Chairman of the Board and Director |
|
March 20, 2023 |
|
|
|
|
|
|
|
/s/ Abraham Bassan
Abraham Bassan |
|
Director |
|
March 20, 2023 |
|
|
|
|
|
|
|
/s/ Jerel Davis, Ph.D.
Jerel Davis, Ph.D. |
|
Director |
|
March 20, 2023 |
|
|
|
|
|
|
|
/s/ Kristen M. Hege, M.D.
Kristen M. Hege, M.D. |
|
Director |
|
March 20, 2023 |
|
|
|
|
|
|
|
/s/ Joseph Jimenez
Joseph Jimenez |
|
Director |
|
March 20, 2023 |
|
|
|
|
|
|
|
/s/ Matthew Porteus, M.D., Ph.D.
Matthew Porteus, M.D., Ph.D. |
|
Director |
|
March 20, 2023 |
|
|
|
|
|
|
|
/s/ Carlo Rizzuto, Ph.D.
Carlo Rizzuto, Ph.D. |
|
Director |
|
March 20, 2023 |
|
|
|
|
|
|
|
/s/ Smital Shah
Smital Shah |
|
Director |
|
March 20, 2023 |
|
|
|
|
|
|
|
/s/ Jo Viney, Ph.D.
Jo Viney, Ph.D. |
|
Director |
|
March 20, 2023 |
|
|
|
*By: |
/s/ Josh Lehrer |
|
Josh Lehrer, M.D. Attorney-in-fact |
149
150
Similar companies
See also AMGEN INC - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)See also GILEAD SCIENCES, INC. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also Moderna, Inc. - Annual report 2022 (10-K 2022-12-31) Annual report 2023 (10-Q 2023-09-30)
See also BioNTech SE
See also BIOGEN INC. - Annual report 2023 (10-K 2023-12-31) Annual report 2025 (10-Q 2025-03-31)
